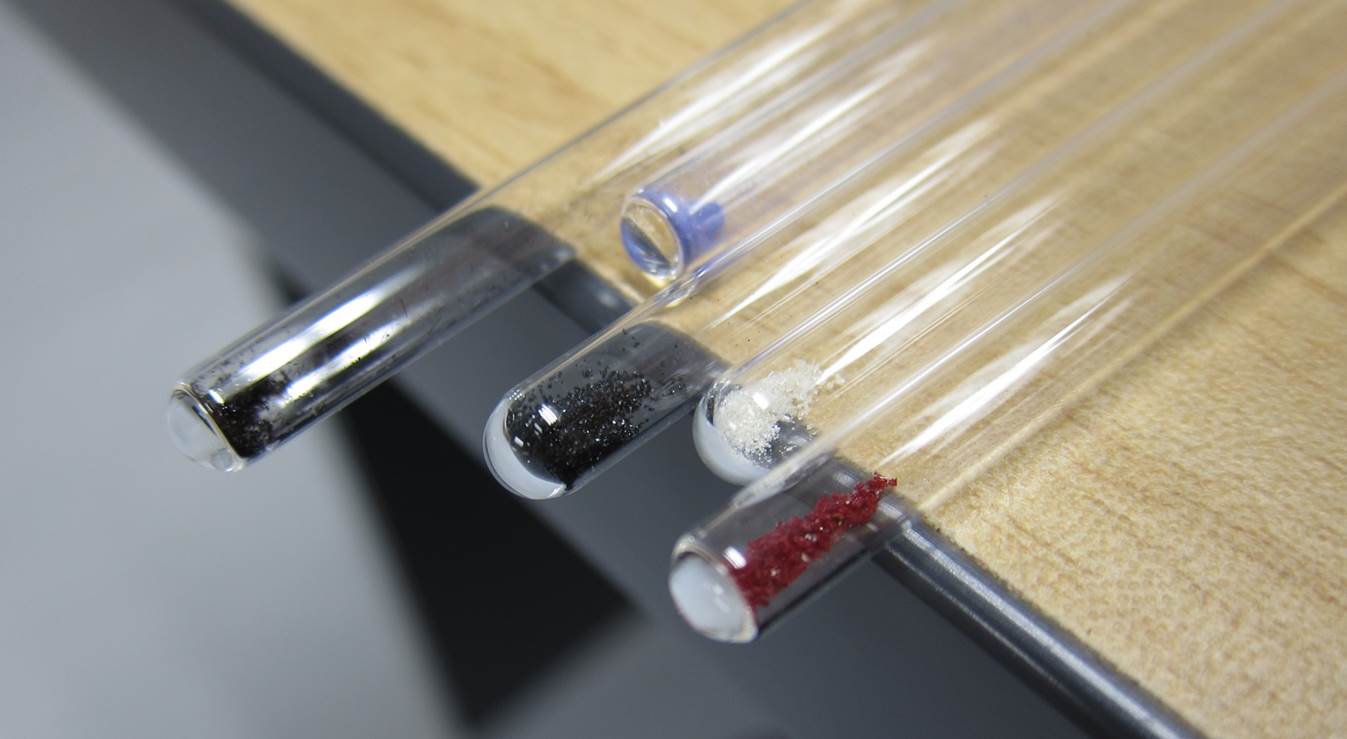
We have put together a new lab manual, ‘Preparation and Characterisation of Metal Acetylacetonate Complexes’ that exposes students to a range of concepts including keto-enol tautomerisation, Evans method, metal complex synthesis and electronic structure.
In this experiment students synthesise paramagnetic and diamagnetic metal acetylacetonate complexes. Acetylacetonate is a common ligand which binds through both oxygen atoms to the metal centre, forming crystalline solids that are soluble in organic solvents. The Paramagnetic protocol of the Spinsolve is used to obtain spectra of these complexes and determine whether they are diamagnetic or paramagnetic. For paramagnetic complexes, Evans method is used to determine the magnetic susceptibility of the complex and therefore the number of unpaired electrons and electronic structure of the complex.
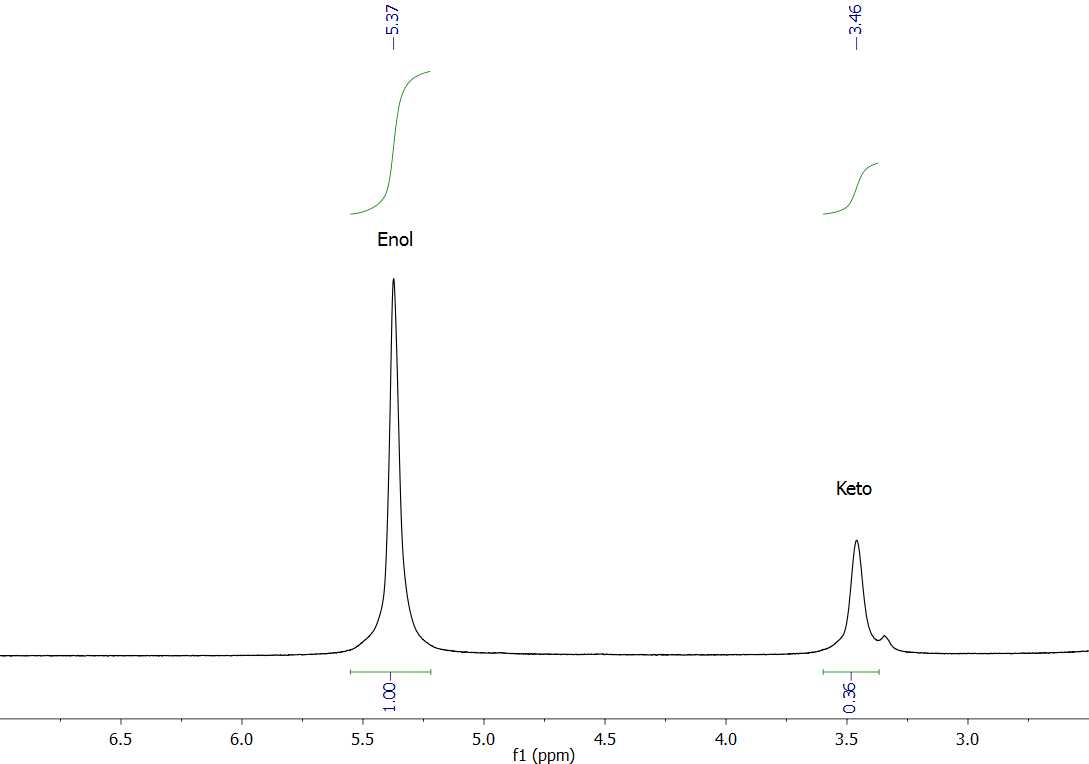
The proton NMR spectra of diamagnetic complexes resembles that of the enol tautomer, thus comparison is used to identify the peaks responsible for each tautomer. The 1D proton spectrum is then used to determine the keto:enol ratio of acetylacetone in chloroform.