Spinsolve 60 ULTRA Multi-X at University of Mons – Center of Innovation and Research in Materials and Polymers (CIRMAP)
“The benchtop NMR Spinsolve represents an incomparable work tool, and the choice of functionalities is vast. It's certainly one of the best investments I've made in a long time.”
Prof Coulembier is Senior Research Associate for F.R.S. – FNRS & AXA Professor in Chemistry at the Center of Innovation and Research in Materials and Polymers (CIRMAP) of the University of Mons (Belgium). His research group is centered around macromolecular engineering by biomimetic inspiration, focusing on developing strategies to control the preparation of complex macromolecular topologies through the design of new monomers, initiators, and on-demand catalysts. Their efforts emphasize sustainability in polymer science, including 'green' methodologies for polymer synthesis and strategies to address plastic waste and valorize carbon dioxide. To drive chemical discoveries towards application, they collaborate closely with engineers, biologists, and medical professionals across academia and industry.
As the supervising professor overseeing a dedicated PhD student's work on CO2 valorization, he has directly observed the transformative impact of our research. Our project focuses on synthesizing carbonate salts by bubbling CO2 in presence of alcohol and an organic base, with the goal of optimizing synthesis conditions and understanding their degradation over time. However, we faced significant challenges, including the instability of the synthesized salt and the limited access to high-field NMR, which had to be shared among various research groups within the university, allowing us access a very limited access. The extensive sample preparation required — involving the evaporation of the reaction solvent and subsequent redissolution in a deuterated solvent — further complicated our efforts.
Introducing the benchtop NMR Spinsolve in our lab marked a pivotal advancement. Placed conveniently next to the reaction vessel, this state-of-the-art instrument allowed for direct characterization of reaction products without the need for extensive sample preparation. The no-deuterated solvent (no-D) NMR analysis coupled with efficient solvent suppression pulse sequences streamlined our workflow, saved valuable time, and significantly improved the accuracy and reliability of our results.
With the Spinsolve benchtop NMR, we were able to monitor the synthesis and degradation of the prepared salts in real-time, providing critical insights that were previously unattainable. This technological advancement enabled us to overcome instability issues, optimize synthesis conditions more effectively, and accelerate the pace of our research. It has been instrumental in advancing our sustainability goals and expanding the frontiers of innovative chemistry.
I highly recommend the Spinsolve system for any research team looking to enhance their analytical capabilities and achieve greater precision in their work.

Spinsolve 60 at the Friedrich Schiller University Jena
Our group is active in a broad field of applications associated with polymer research. Besides medical and battery applications, the automation of polymer research is also one of our major concerns. Using synthesis robots, we investigate different types of polymerizations. In this context, the monitoring of reactions, in particular polymerizations, e.g., regarding the degree of conversion of the monomers, is of crucial importance. Prior to the use of the Spinsolve instrument, samples were taken from the reactors and had to be measured offline using high-field NMR spectrometers. Applying this type of sampling, the question of comparability sometimes arises due to the remaining time between sampling and measurement. Using the new Magritek Spinsolve instrument, we are able to acquire and evaluate the reaction results directly and without any delay in continuous flow with sufficient sensitivity and resolution.
The purification of the polymers also takes up an important part of our research. By using the Magritek Spinsolve, we were able to improve this process step. Once more it offers the possibility to directly monitor the process of purification. Consequently, it was possible to determine several factors affecting the overall purification efficiency.[1,2] This process would have been very tedious to nearly impossible with high-field NMR equipment.
Reference:
[1] T. Schuett, I. Anufriev, P. Endres, S. Stumpf, I. Nischang, S. Hoeppener, S. Zechel, U. S. Schubert, R. Geitner, “A user-guide for polymer purification using dialysis”, Polym. Chem. 2022, 14, 92–101, DOI: 10.1039/D2PY00972B.
[2] T. Schuett, R. Geitner, S. Zechel, U. S. Schubert, “Dialysis diffusion kinetics in polymer purification“, Macromolecules 2021, 54, 9410–9417, DOI: 10.1021/acs.macromol.1c01241.

New Spinsolve at Nantes University – CEISAM Laboratory
Dr. Jonathan Farjon is a French CNRS researcher from CEISAM lab at Nantes University. He is involved in the development of NMR methods for improving the performance of benchtop apparatus. Since 2018, he has led different projects involving benchtop NMR for in-line flow reaction monitoring, food transformations, and microalgae cultivation bioprocesses. Pioneer results were achieved on the three Spinsolve systems of the lab thanks to the development of NMR approaches to overcome limitations related to the reduction of the magnetic field on compact NMR instruments.
For tracking New Psychoactive Substances (NPS) that are becoming a threat to public health, it becomes urgent to precisely characterize NPS. Thanks to a project led by Jonathan Farjon, between CEISAM and the scientific police department of Lyon, and involving Thomas Castaing-Cordier as a Ph.D. student, one more step was reached with an analytical platform that features a Spinsolve 60 MHz equipped with an autosampler.
In order to improve the performance of the Spinsolve 60 MHz, CEISAM has implemented a set of optimized 1D and 2D NMR experiments on this compact spectrometer. By combining these approaches with Infrared spectroscopy, a multi-method database was created to identify and also, for the first time, elucidate the composition of seized drugs. This integrated analytical platform was blindly validated on 6 real samples seized by the scientific Police of Lyon. In addition, NMR quantification was used to assess the purity of each NPS, allowing for better tracing of trafficking networks.
This transportable multi-method platform will provide the Police and more broadly forensic laboratories with affordable routine analysis tools. These actors will be able to identify and even elucidate new drugs without the need of an exact mass determination, usually obtained by high resolution mass spectrometry which remains expensive.
Reference
Castaing-Cordier, T.; Benavides Restrepo, A.; Dubois, D.; Ladroue, V.; Besacier, F.; Buleté, A.; Charvoz, C.; Goupille, A.; Jacquemin, D.; Giraudeau, P.; Farjon, J. Characterization of new psychoactive substances by integrating benchtop NMR to multi-technique databases. Drug Test Anal. 2022 doi:10.1002/dta.3332

Spinsolve Carbon ULTRA at Corden Pharma
Cordenpharma has installed in Chenove its center of Excellence for flow chemistry. Beside a modular GMP pilot line for flow chemistry, this includes as well a state of the art flow chemistry lab for doing feasibility studies (proof of concept), or process development. State of the art does not mean flow reactors only, but an infrastructure which allows to exploit all advantages of flow chemistry. One important part of this infrastructure are spectrometers for online measurements( NMR, IR, Raman, UV-Vis etc.), which is a key element to perform rapidly and efficiently feasibility studies and process development. In this context the Spinsolve ultra, has proven extremely useful. . It is in comparison with the IR and Raman spectrometer, which require some work to find peaks to monitor, the first choice in most of the flow experiments as it delivers with a minimum preparation the max information. It has high stability, is robust, and easy to use. In case of a perfect flow setup, the NMR permits screening reaction conditions using Design of Experiments (DoE) in a very fast way ( 10-20 ( or more) reaction conditions a day).

Spinsolve Nitrogen at Universitätsklinikum Schleswig-Holstein
“The Spinsolve is a great machine! It allowed us to implement a highly automated, mostly 3D printed magnetic-field-cycling device for our experiments with hyperpolarized substrates. The support of Magritek was absolutely essential, uncomplicated and great!“
Prof. Dr. rer. nat. Jan-Bernd Hövener

New Spinsolve at Aspen Oss B.V.
Last week, a new NMR system arrived at the Analytical Sciences Department of Aspen Oss. NMR stands for Nuclear Magnetic Resonance, which is a powerful technique to qualitatively and/or quantitatively research materials from solvents to APIs.
This latest addition is a low-field NMR with a field strength of 60 MHz. This new NMR enables Aspen API to bring this powerful analytical technique inside our factories. The system is small enough to fit on a bench-top or inside a fume hood. However, it has very high sensitivity and sufficient resolution for various at-line analyses.
We are very excited about our new NMR system because it will help our production facilities to further improve their efficiency and reliability with very fast turn-around times for the analyses and a very high accuracy. Additionally, the technique is very operator friendly, which means that QC-technicians or production personnel will be able to generate the data they need, to facilitate smooth production processes and batch releases.
This low-field NMR is a great addition to our existing 500MHz NMR platform. This is the platform that we currently use for batch releases under cGMP and for our support to Aspen API’s R&D activities. On this platform, we have the flexibility of measuring liquid samples, solid state sample, and various types of 2D NMR at high resolution and sensitivity. However, the 500MHz NMR platform is immobile, and only highly qualified personnel can handle it. This is where the 60 MHz NMR comes in!

Spinsolve 80 at University of Applied Sciences Aachen
After more than ten years’ experience with high-field NMR equipment, I am convinced that this technique is a powerful tool for structure elucidation, multicomponent mixture analysis as well as for the purity determination of single compounds. Therefore, after becoming a professor, I was very enthusiastic to show students different possibilities of this technique. Unfortunately, modern high-resolution NMR spectrometers are expensive in terms of initial investment, consumables, maintenance, and operation. Especially in an academic environment, where students do not have much time during practical laboratory courses, the implementation of NMR teaching concepts is difficult. Therefore, we were very happy, when we got an opportunity to buy an 80 MHz Spinsolve system from Magritek.
After one-day installation and accompanied by informative instructions, it took only about three weeks to integrate NMR spectroscopy in the practical course of instrumental analysis for undergraduate students. Moreover, due to its compact size, fast measurements, and user-friendly interface, it is now possible to use the spectrometer during lectures and seminars. For example, with master students we follow 1D and 2D NMR measurements of different monomers and polymers and solve some analytical challenges. And this is without using deuterated solvents and nitrogen cooling! Here it should be mentioned that Magritek support is always very fast and objective. We are very pleased to have such a neighbor.
Besides teaching, Benchtop NMR devices offer an uncomplicated integration of NMR measurements into various research projects. Together with our partner University of Applied Sciences (H-BRS) we developed an NMR method for determining lignin’s molecular weight and found the possibilities to transfer multivariate regression models between high-field (600 MHz) NMR and benchtop NMR devices (43 and 60 MHz). Two bachelor students are currently working on the development of an analytical method for the holistic quantification of Aloe vera extracts. We look forward to having more experience with this exciting system!

Spinsolve 43 with active 13C/129Xe switch on the X-channel at EPFL, LIFMET
Dissolution Dynamic Nuclear Polarization (dDNP) is with no doubt the most versatile technique to enhance the NMR sensitivity in the liquid state. In principle, all NMR nuclei can be hyperpolarized by dDNP. DDNP is a central research topic at LIFMET. By the end of 2020 I received funding for the project “The DNP Warehouse: Hyperpolarized Magnetic Resonance for all”, with the aim to increase the dDNP throughput both on-site (multi-sample and multi-nuclei capability of the polarizer) and off-site (making hyperpolarization transportable). The Spinsolve 43 with active 13C/129Xe switch on the X-channel, was the perfect complement to this project. It’s portability, reliability and ease of use allow us to estimate the signal enhancement and relaxation time of our hyperpolarized samples in different experimental situations.
For instance, we can couple our newly built three samples dDNP probe to the Spinsolve 43 and automatically acquire, upon successive dissolutions within 20 min, the hyperpolarized signal of water protons (angiographic application potential), 13C labeled glucose/pyruvate (real time metabolism investigation potential), and 129Xe gas (lung imaging and perfusion potential) using the same benchtop spectrometer with no need to change/tune the NMR probe. This work will be presented during ISMRM 2022 by Mr. Thanh Lê.
Differently, we can move the Spinsolve far away from the polarizer to perform a dissolution “off-site” and use it to estimate polarization losses after extraction and transport of a hyperpolarized sample (Capozzi et al, Nature, Communication Chemistry 4, 95 (2021)).
Besides the well-known high-quality features of the Spinsolve, we were the first group who had the chance to test the active switch on the X-channel. We are very happy, and we can witness that it is a superior tool if the multinuclear capability is required.
Andrea Capozzi, Ph.D.

Spinsolve Nitrogen ULTRA at Helmholtz Institute Mainz
Our group at Helmholtz Institute, Johannes Gutenberg University Mainz is working on novel applications of hyperpolarization for enhanced NMR spectroscopy and imaging. Given the complexity of our experiments, we needed a benchtop spectrometer which (1) is easy to set up for various trigger and acquisition schemes, (2) has the ability for designing custom-made pulse sequences, (3) has a great homogeneity to distinguish chemical shifts at ~1 T magnetic field. I can confidently state that among several brands of benchtop NMR spectrometers I had a chance to operate, Magritek stands out as supreme in all these categories. In our latest work (see the picture) we combine a fully automated gas bubbling system and a robotic arm setup to realize polarization transfer at a selected magnetic field with subsequent transfer of the sample to the spectrometer for spectral acquisition. All of these steps were relatively straightforward to realize using the Magritek spectrometer and resolution exceeded my initial expectation. Taken together, I highly recommend Magritek for anyone who wants to put their money into reliability, stability, and performance.

Spinsolve 80 ULTRA at VITO (Flemish technological research institute)
VITO NV (Flemish technological research institute) is actively involved in many activities concerning the sustainability of materials and processes. As a part of the development of the MESCH (membrane processes) team and SPOT (Sustainable Polymer Technologies) team the use of spectroscopic techniques for the characterization of samples is very important. So far we were outsourcing NMR experiments. Due to the high volume of samples we decided to purchase a benchtop NMR magnet. After the successful trials conducted by Magritek, that meet all our expectations, we didn’t hesitate to buy the Magritek Spinsolve 80 Ultra 31P.
31P measurement is of crucial importance for SPOT team because one of our core research areas is lignin materials development. 31P is used to characterize lignin functionalities. We were impressed with the performance of the 80 MHz compared to the 400-500 MHz magnets, so much that we decided to publish the results in lignin field related journal - Industrial crops and products.1
On other side, 1H proton is of great help for our developments as well. We work also with small building blocks for biopolymer synthesis. The resolution of the 1H spectra with the 80 MHz benchtop NMR is very good, which allows us to make an assessment of sample’s purity and reaction conversion. Which is also crucial for the development of other teams at VITO.
Magritek NMR is very user friendly, with no maintenance at all compared to big magnets. We ordered the autosampler accessory as well which is a must if you have many samples to be measured in a single day.In addition, the software is very easy to use and to get familiar with it, especially important for students/interns joining VITO.
Last but not least, we would like to highlight that Magritek support has been great, solving each question we had regarding the capabilities of the NMR before and after the purchase of the benchtop NMR.

Spinsolve at CNRS, Chimie ParisTech

The implementation of the Spinsolve 60 spectrometer directly in our lab allows us to analyze our samples in less than one minute after their preparation, which is extremely convenient. We recently obtained the 1H NMR signatures of several sensitive organoiron species (less than 5 minutes lifetime); the resolution reached by the Spinsolve 60 led to publishable spectra, with a very good signal-to-noise ratio on a large 500 ppm spectral window. This spectrometer is definitely a strong asset in our research activity.

Spinsolve 80 at KIT – Karlsruhe Institute of Technology
In our group at KIT we are working – among others – on the combination of analytical methods, e.g. separation methods in combination with spectroscopy technologies. In one project we combine GPC, sometimes also called SEC, with table-top NMR spectroscopy. One of the advantages of the Spinsolve NMR spectrometer from Magritek is its capability to measure samples in flow. With this combination, we have built up a two-dimensional characterization GPC-NMR, with molecular size resolution for polymers due to the GPC and chemical information due to the NMR.
This project is very much demanding, especially for the Benchtop NMR part. As it is well-known, the sample coming from GPC is in low concentration and therefore our NMR spectrometer has to be very sensitive. For this purpose, Magritek has tuned our Spinsolve 80 to the highest possible S/N via a special “proton only version”. It is also required to work with flow cells that allow a maximum of sample within the NMR coil – here the Spinsolve has the big advantage that it has an external hardware lock system integrated. We have conducted substantial optimization on the flow cells. Thus the sample amount into the NMR coil for the flow cell is comparable to a 5 mm standard NMR tube and a deuterated sample is not required to lock the NMR.
Other advantages of the Spinsolve have found to be the high spectral resolution of the spectrometer which helps in our application and for the solvent suppression methods. By using a flexible Magritek software surface (Expert Software), we can build our own methods and even pulse sequences.
Overall we can state the besides the high performance and quality of the Spinsolve spectrometer, Magritek has always been very supportive when we were asking for special adaptations and when support has been required in general.
In the meantime we could publish the results of this combinational method in several papers:
- Höpfner, K.-F. Ratzsch, C. Botha and M. Wilhelm, Macromolecular Rapid Communications, (2018): Medium Resolution 1H-NMR at 62 MHz as a New Chemically Sensitive Online Detector for Size-Exclusion Chromatography (SEC–NMR)
- Botha, J. Höpfner, B. Mayerhöfer and M. Wilhelm, Polymer Chemistry, (2019): On-line SEC-MR-NMR hyphenation: optimization of sensitivity and selectivity on a 62 MHz benchtop NMR spectrometer
- Höpfner, B. Mayerhöfer, C. Botha, D. Bouillaud, J. Farjon, P. Giraudeau, M. Wilhelm, Journal of Magnetic Resonance, (2021): Solvent suppression techniques for coupling of size exclusion chromatography and 1 H-NMR using benchtop spectrometers at 43 and 62 MHz

Spinsolve at B4Plastics


Spinsolve at Zürcher Hochschule für Angewandte Wissenschaften Institute of Materials and Process Engineering
Our group, the Laboratory for Polymeric Coatings, belongs to the Institute of Materials and Process Engineering from the Zurich University of Applied Sciences. We are generally engaged with functional, smart, and monomolecular coatings, but also with classical coatings on materials such as paper and cardboard, textiles, metals, and plastics. To this end, synthesis of small molecules (monomers) and novel polymers is often needed. Organic Synthesis without an NMR-spectrometer is unthinkable nowadays. Because our lab. space and resources are limited, we outsourced our NMR spectra to an external lab., equipped with a high field NMR spectrometer. However, the loss of time became unbearable. Hence, we investigated the acquisition of a benchtop NMR Spectrometer. We evaluated many instruments and finally choose the Spinsolve 80 MHz Carbon Ultra from Magritek.
We are very pleased with this instrument. It covers most of our needs. Especially noteworthy are the narrow line shapes, the intuitive software, and the competent and fast service from Magritek. We can thus recommend this instrument and Magritek.
Dr. Konstantin Siegmann

University of Applied Sciences and Arts Northwestern Switzerland
As an engineering institute we are developing in the field of polymeric materials new products and processes from the concept phase, via synthesis, processing, testing, and recycling together with our industrial partners. To follow and understand changes at all steps, we always need to be able to detect, what is happening with our materials during processing. Hence, we have several analytical techniques including UV-VIS, IR, Raman spectroscopy as well as thermal analysis, GPC, SEM. However, only in combination with the powerful technique of NMR we can confirm the chemical structure and changes of our monomers, polymers, and additives on a molecular level.
Thanks to the ease of use, the low maintenance cost, and the high flexibility with three nuclei 1H, 13C and 19F and several 1D and 2D experiments the Magritek Spinsolve 60 MHz Carbon is used in many different projects and besides the confirmation of the chemical structure we also measure the reactivity in polycondensation reactions as well as the composition of copolymers. It would have never been possible for us to buy and maintain a high field NMR due to the needed personnel as well as to the high cost. Due to the user-friendly software and the possibility to program via macros several experiments in series, the measurement time can be used in a very efficient manner. Thanks to the tabletop device from Magritek we are able to extensively use this very versatile technique and to integrate it into the curriculum of our students

Spinsolve at Zschimmer & Schwarz
Zschimmer & Schwarz is a family-owned chemical company offering a broad portfolio of solutions to customers formulating products for various end-user markets, like Homecare, Industrial applications, Personal Care, Construction, Automotive, Leather or Paints and Coatings – just to name a few.
One of our core competencies is the customisation of products as the motto of the company says: Chemistry Tailor Made. Our self-conception of customisation includes the understanding of processes on a molecular level, as we want to offer our customers the most powerful and safe products.
State-of-the-art analytical devices form the backbone for this claim. In the field of NMR technology, we have chosen Magritek as preferred partner. At Magritek we found the ideal package consisting of support from the Magritek-team, user-friendly equipment, and cost-effective operation. Our analytical portfolio of three Spinsolve 80 NMR spectrometer enables us to study compounds possessing 1H, 19F, 13C, 29Si and 31P nuclei. Both areas, R&D and production / quality control, use the high-quality data provided by the spectrometers. In addition, the use of the autosampler extension shortens our time to market for R&D and customising projects as it allows quick and standardised analysis of samples.
Based on our experience, Zschimmer & Schwarz looks forward to a fruitful cooperation with Magritek in the future.
Dr. Philipp Kitschke & Dr. Bernd Schlichting

Spinsolve at Halag Chemie AG, Aadorf, Switzerland.
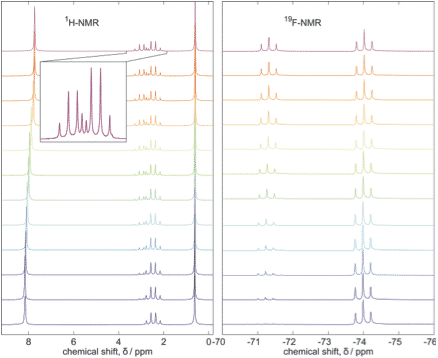
The tabletop Spinsolve 60 Carbon Ultra NMR drastically improved our analytical possibilities. We mainly use the 1H-Proton measurement for quality control. Our products are mostly water based, but strongly corrosive (acidic, alkaline). NMR with its contact free measurement is very well suited to our needs.
Owning and running an NMR spectrometer would never have been possible to us if it still needed cryogenic cooling. The Spinsolve has a small footprint and low maintenance costs.
In the evaluation phase, Magritek helped us with measurements of more than 50 samples to find out, which Spinsolve model best fits our needs. We even visited Magritek in Aachen for a live demonstration. Once delivered, we did not need special instructions to set up the spectrometer. The manual is user friendly and intuitive to follow. Magritek was readily accessible for assistance, although due to the simple installation procedure, our only questions centered on minor topics regarding software and system logic.
With efficient and reliable solvent suppression, we are able to detect even the smallest differences in our samples. A short 60 seconds scan (no shimming needed) is usually enough to tell the formulations of our factory apart. We reach a sample throughput of up to 40 samples per hour.
In addition, we run 13C NMR, 19F-NMR, or 2D-NMR on specific compounds for special projects.
After one year of use, the signal strength and the signal to noise ratio remains stable which is an important requirement for proper quality control.
Magritek delivers what they promise. A precise and robust NMR spectrometer that is user-friendly and fast. The customer support has a short reaction time, is uncomplicated and friendly from the first contact until today.

Spinsolve at Furtwangen University Faculty of Medical and Life Sciences
To reach excellent teaching in interdisciplinary disciplines like medical and life sciences knowledge in the wide field of chemical and scientific analysis is mandatory. Structure determination of small molecules, quantification of metabolomics and reaction monitoring are only a view to mention, but without proper equipment it is often hard to fulfil the requirements of teaching.
In the past, NMR spectroscopic teaching could only be covered in theory and in chemical research projects we had to depend on institutional cooperation partners for NMR measurement, what led to time consuming processes.
With our new Spinsolve Carbon we are now able to provide practical NMR spectroscopy to our students and in chemical research we can perform quick and fast analysis of small compounds leading to a much higher efficiency in teaching and research.
Prof. Magnus Schmidt has just published his first article using the Spinsolve and the publication can be downloaded here:
Online - CLICK HERE
PDF - CLICK HERE

Spinsolve at the Quality Control Steinheim Sigma-Aldrich Chemie GmbH
Quality Control in Steinheim
Since we have limited space in our quality control department, we have never had the opportunity of operating an NMR device on site. After getting to know the Magritek Benchtop NMR for reaction monitoring during my studies at university, we implemented this comparably very small and easy-to-use device in our quality control lab. We mainly use it to confirm the identity of pure chemicals, but also determine possible impurities in certain cases. Besides being able to perform the measurements on site to save time and money, our lab personnel get first-hand experience and knowledge about the state-of-the-art NMR analytic which would not have been possible otherwise.
Overall, we are very pleased with this instrument and are excited to follow future developments in the field of Benchtop NMR technology.

University of south Brittany, Lorient, department of LBCM
Fabrice Azemar is active in (co)polymers synthesis and modifications for applications in antifouling coatings. There is no high field instrument on his site. Before he had the Spinsolve, the samples for NMR measurements were sent to the university of Brest, which took him 3 to 5 days to get the results. An alternative was to drive (about 150 km) with the samples to the NMR facilities. Now, the spectra are directly acquired in the lab, he gains a lot of time and money. “A synthesis that took previously 3 weeks, can thanks to the Spinsolve be done in a couple of days” says Fabrice Azemar.
The instrument allows him and his team to have quickly a confirmation of the success of the reaction and in most of the case quantitative results. Some of them were already published in the journal “Polymers” (DOI: 10.3390/polym10040445 ). Thanks to the easy to use software, after a short training of less than 20 min, students, interns and PhD students can use autonomously the instrument.

School of Pharmacy at Temple University
In order to ensure that our students are fully prepared to tackle the most pressing research problems of the next generation, we must ensure that they have the most advanced technology possible. The arrival of the Magritek Spinsolve in our institution is yet another example of Temple’s commitment to its students and high quality research.
“Students, post-doctoral scientists, and visiting faculty have access to the benchtop Spinsolve NMR Spectrometer, allowing rapid, hands-on acquisition of critical program data, enabling them to solve complex research problems in a more efficient manner.”
….the sleek benchtop design of this instrument will greatly simplify its integration into the existing laboratory space of the school of pharmacy. Its ease of use is expected to significantly enhance productivity of students and faculty, providing them with greater opportunity to pursue advanced research on a highly competitive level. Students, post-doctoral scientists, and visiting faculty have access to the benchtop Spinsolve NMR Spectrometer, allowing rapid, hands-on acquisition of critical program data, enabling them to solve complex research problems in a more efficient manner. The system will be a focal piece of the Moulder Center for Drug Discovery Research’s analytical technology suite.

Rochester Institute of Technology
We chose the Spinsolve NMR for use in an Instrumental Analysis laboratory with 90 students (mostly chemical engineers). In addition to NMR, students get exposure to UV-Vis, fluorescence, AA, and IR spectroscopies. We wanted an NMR with a low operating cost which could be sited in the lab, and leave the students with a positive NMR experience. Just like with the Terranova from Magritek, the Spinsolve scores high points for ease of use, both in collecting and processing the data. Shimming is easy and does not need to be performed on each sample. The resolution is sufficient for students to easily analyze 1- and 2-D H spectra of small molecules. It can be used to measure spin-lattice and spin-spin relaxation times at 40 MHz that we cannot take with other instruments.
Just like with the Terranova from Magritek, the Spinsolve scores high points for ease of use, both in collecting and processing the data.

Wageningen University & Research, The Netherlands
Students are taught the basics of NMR and in small groups work one afternoon with the Magritek 60 MHz NMR. They analyse the alcohol content of various liquors (quantitative NMR). In the same course, students also isolate some products (anethol, piperine, xanthorrhizol) and can then measure their own product to evaluate the identity and purity. In addition to the 60 MHz spectra, 400 MHz spectra are handed out. Immediately after this course, there is an organic synthesis course where students synthesize products. Again afterwards, they assess how successful their synthesis was. So they apply the knowledge acquired earlier.
In the 45 years before the acquisition of the Magritek, we used our research NMR (60 ? 90 ? 200 ? 300 ? 400 MHz) for teaching purposes. However since our move to a new lab and the sharp increase in the number of students, this has become logistically impossible. However we did not wish to lose the hands-on aspect and we like students to measure their own products. Therefore, we had to find an affordable alternative solution, i.e., an instrument with a low-field permanent magnet. Although the field strength poses a problem (we can no longer use certain compounds as the spectra are too complicated at 60 MHz), we chose 60 MHz. We tested four different table top low-field NMR instruments, and the Magritek came out best in terms of line width, sensitivity and ease of operation. The price was in the same range as the other instruments so we bought the Magritek. The Magritek also allows for all 2-dimensional (2D) NMR spectra but so far we have not used this option as the introductory course does not deal with 2D NMR. However it is possible, that we will use this option in one of the more advanced NMR courses.

Long Beach City College
Previous to adding NMR capability, we introduced our students to IR spectroscopy and gas chromatography. When we saw the performance and price of the Spinsolve Benchtop NMR spectrometer from Magritek, we saw our opportunity to fulfil another of our goals. In particular, we were impressed with the durability and robustness of the machine. The range of different applications such as the 2D capability was most important when we came to make our purchase decision.
“We were impressed with the durability and robustness of the machine. The range of different applications such as the 2D capability was most important when we came to make our purchase decision.”
See the blog post here.

University of Queensland
Students used to miss the link from theory to practical use. Now my groups can have a hands-on experience and understand the value of NMR to the synthetic chemist. My second semester first-year students are taken through the basics of using Spinsolve. They make a product (paracetamol); run it on the NMR; acquire and process the data. Some of our more high achieving undergraduate students go a stage further with a six week research project where they are introduced to spectroscopy – IR, NMR and GC-MS. Each student is allowed to choose their own project. For example it could to study different reactions of benzaldehyde. They will analyse the products they synthesise to look at variations in substitution products. They are taught to use multiple techniques to confirm what they have made and the Spinsolve NMR machine is key to achieving this.
“The versatility of Spinsolve is just great. It gives us the flexibility we need. Students and staff alike are very pleased to have access to it.”
See the blog post here.

Durham University
"It has a small footprint; it is robust (student friendly); and is inexpensive in comparison to purchase and operating costs for a high field NMR. In the near future, we are going to place this in series with our Syrris Africa Microfluidic System for online NMR of new chemical entity library generation under flow conditions. This would be tandem with online LC/MS and would give the opportunity to have very fast synthesis and analysis of hundreds of compounds overnight. Setup of Magritek will be very easy in comparison to a high field, supercooled NMR."

Durham University
being able to do measurements in the relatively low magnetic field (43 MHz) used by Magritek’s Spinsolve is a big advantage for us, particularly as the field it uses it not very different to the field actually used in many hospital MRI scanners.
…real time reaction monitoring using flow-through NMR techniques. This is not a new idea, but has previously been limited by the requirement to bring the reaction close to a large (static) and expensive NMR spectrometer. The small size of the Spinsolve means that we can mount it on a trolley with an uninterruptible power supply (UPS) and move it around the department – taking the spectrometer to the fume cupboard where the reaction is being carried out. Working with partners in industry, we are evaluating this in parallel with small footprint Mass Spectrometry to see what relevance this approach might have not just in the University laboratory but also in pilot plants and possibly even at production scale.
…the opportunities for students to get involved “hands-on” become less, so exposing them to a robust, simple instrument they can use themselves is a big plus.
See the blog post here.

College of Humanities and Sciences at Virginia Commonwealth University
Meredith Moses teaches a hands-on approach:
We use the NMR so that the students know and understand about NMR. If they go to grad school or industry, they need to be familiar with it. Also, they are expected to be able to analyze spectra for their second semester (lecture) final exam. I like them to produce spectra of their own compounds so they know if they got the right product or not. Some instructors just hand out spectra of one groups’ sample or use literature spectra, but that somewhat defeats the purpose of a practical course. Students should know how good THEIR product is.
“I like [students] to produce spectra of their own compounds so they know if they got the right product or not. Some instructors just hand out spectra of one groups’ sample or use literature spectra, but that somewhat defeats the purpose of a practical course.”
See the blog post here.

Howard School of Arts and Sciences at Samford University, Birmingham
the greatest benefit of the system is its ease of use and low-level of training for student operation. We are also very fond of no longer needing an air source for spinning. The instrument is very fast and reliable compared to the old, larger, 60 MHz instrument we had. The requirement of consistent spin and the finicky nature of the software made the instrument less practical for student use, and very challenging for research purposes.”
“The greatest benefit of the system is its ease of use and low-level of training for student operation.
With the simultaneous 19F and 1H “nested” monitoring scripts, we can get reinforcing data by monitoring the reaction progress via two nuclei instead of just one. Additionally, when the dispersion issue arises during that experiment, we no longer have to abandon the more substituted alcohols because we can simply monitor the reaction via the 19F spectra.

Tarrant County College
Chemistry Department Chairman, Dr Kaveh Azimi, and staff, are enthusiastic about their decision.
Faculty selected Spinsolve spectrometer for many reasons. Its compact size is ideal for a teaching laboratory where space is at a premium. Maintenance free operation thanks to the permanent magnet design means there is no need for cryogen fills saving valuable resources. The simplicity of sample preparation and the fact that the system uses standard 5 mm NMR sample tubes make this an ideal instrument for a teaching lab. The quality of spectra is excellent and to have the option of a 13C upgrade increases the value of this initial investment.
“We were “magnetized” by Spinsolve’s simplicity of operation and the superior quality of spectral output during the hands-on instrument demonstration. The Spinsolve took us for a surprising spin!”
Staff said
We were “magnetized” by Spinsolve’s simplicity of operation and the superior quality of spectral output during the hands-on instrument demonstration (high signal to noise and good peak resolution). The Spinsolve took us for a surprising spin!

Bloomfield College
NMR is essential at many levels of chemistry. It is essential in graduate school and industries such as foods and pharmaceuticals. Providing a foundation in the analysis of simple samples by NMR at the undergraduate level will help prepare my students for the interpretation of the much more complex spectra of more complex molecules. The more hands-on experiences the kids get as undergraduates, the better they are prepared for further studies and/or the workplace.
“The more hands-on experiences the kids get as undergraduates, the better they are prepared for further studies and/or the workplace.”
Purchase costs, prohibitive maintenance bills and complex operating conditions all made the thoughts of obtaining an NMR for undergraduates all but impossible. As she says,
For many years, no one was interested in manufacturing a simple NMR for undergraduate student use. It was all about the big-bucks systems. In recent years, however, I became aware of three or four such instruments becoming available. I looked into them but, quite frankly, was less than thrilled with their performance until Magritek came into the picture. Magritek’s Spinsolve is by far the easiest instrument to use. It is also extremely fast, with an excellent spectrum generated and printed out in a minute or two. The software is intuitive. Over my many years in research and teaching laboratories, I have seen instruments get more and more complex with even more complex software. These instruments are very powerful and can do a lot of stuff. However, they are less than user-friendly. First and foremost, I am interested in teaching my students as much as possible. I am not big on demos. I want the kids to run their own spectra and then analyze the results. Lab work should be fun. Generating and analyzing your own spectrum is a lot of fun. Most students really don’t like solving puzzles. However, I must say that they certainly enjoyed their experiences with the Spinsolve.
“Magritek’s Spinsolve is by far the easiest instrument to use. It is also extremely fast, with an excellent spectrum generated and printed out in a minute or two.”
See the blog post here.

Höhere Technische Bundeslehranstalt Wels
HTBLA Wels is a higher technical vocational college of chemistry in Austria. Here, Dr Harald Baumgartner is responsible for the instrumental analytical laboratory. NMR has been part of the curriculum for some years. However, this was through a very old 1H-60 MHz NMR. New instruments usually require fillings of expensive liquid helium for cooling the magnet. Dr Baumgartner says “Compared to the old 60 MHz spectrometer, the Magritek Spinsolve benchtop spectrometer is so much easier to use. It is software-based so collecting and processing data is quite straightforward. As well as 1H spectra, our Spinsolve allows us to measure more complex spectra including 13C-spectra. Even 2-dimensional experiments are now available to the students.”
“As well as 1H spectra, our Spinsolve allows us to measure more complex spectra including 13C-spectra.”

Professor in the Department of Chemistry at Cleveland State University, Ohio
It is a small system, portable and lightweight. It is quickly ready to use allowing the students to have a real NMR analysis experience (they prepare the samples in standard NMR tubes, add the solvent and record the spectra in similar ways to a research grade NMR). The spectra are of good quality. The software is friendly and, overall, Spinsolve is readily affordable.
The spectra are of good quality. The software is friendly and, overall, Spinsolve is readily affordable.

The George Washington University
“What I like about the Spinsolve and about the software is the high throughput.”
Commenting on why he likes the Spinsolve for teaching, Dr King says,
”What I like about the Spinsolve and about the software is the high throughput. Obtaining the spectrum takes only a few minutes – less time than making the solution and putting it into the NMR tube. Place the sample tube in the instrument; then run a few scans, we typically do 16, and the students have a high quality spectrum. In terms of preparation we just run a short few minute shim at the start of lab session and it’s good for the rest of the day.”

École normale supérieure de Lyon, France
we are indeed very happy with the experience we’ve had so far with your instrument. We tried to use it as much as we can in our training, and we had the opportunity to offer access to the machine to other students (from highschools and french “preparatory classes” ie 1st-2nd year of academic studies).The ENS Lyon offers a specific training to its students who envision becoming future university teachers. This training involves a lot of laboratory classes. During these classes, our students are requested to present and discuss a variety of synthetic and analytical techniques in front of a jury. So far, for obvious technical reasons, NMR was not routinely available for such presentations. The purchase of the Spinsolve system has been a great improvment in this framework. It has opened new perspectives in the construction of these presentations, and consistently enriched the ensuing discussions (about the nature of the magnet, the information we get from the spectrum regarding the nature and purity o fthe sample, and the main spectral differences obtained as compared with the larger fields cryomagnet spectrometers they have been used to during their internships in research laboratories) . The fact that it can be purchased as an affordable price and virtually no maintenance cost is ideally suited for a teaching department, were running costs are sometimes difficult to handle!

Department of Chemistry at University of Helsinki, Finland
Now all students run their own NMR spectra. They mainly run 1H spectra from their synthesis products. If they are interested, they can also run other spectra (13C and 2D-experiments). Our motivation for using Magritek Spinsolve is to teach our students NMR spectroscopy as a tool to analyze the structures of their synthesis products. The students can also use NMR in addition to IR to solve the unknown organic compounds when they do their qualitative organic analysis work.
The students like to use the Spinsolve spectrometer as it is fast and quite easy to use. For teaching staff, one advantage of the Spinsolve is that it requires very little maintenance and does not require cryogens nor require much bench space. The level of success is reflected in that even some of our researchers have used the Magritek system to look at their reaction products.

Yale-NUS College, Singapore
We have designed our science classes in a way that makes them fairly indistinguishable from actual research.
Dr Presolski’s classes are quite small – fewer than 18 students per class. This means that professors are able to give tailored instruction to their students and provide the key skills needed to properly use the scientific equipment.
You are taught not only how to operate the machines, but also the physical principles behind the measurements and ways to interpret the data without reliance on the default software

Chemistry Department at Lyon College, Arkansas
Spinsolve has become the most popular among faculty and students of our chemistry program. It is used in organic chemistry, instrumental analysis, and advanced inorganic chemistry laboratories. We plan to extend the usage to other areas too. Students have become very interested in the concept of NMR because of this instrument. Their knowledge of NMR is improved tremendously after the incorporation of Spinsolve in the chemistry program. It is easy to operate, provides quick analysis, and requires very low maintenance. Spinsolve is definitely the best fit for a small college like ours. We formerly had a cryogenic NMR spectrometer at Lyon but the chemistry program has not been able to maintain the instrument in the long term. Spinsolve is low cost and its low maintenance is key for its great fit to Lyon chemistry program. It is also used in recruiting keen students as they get really excited about the instrument and its capabilities during frequent campus tours.
Then she adds a few words about the use of Spinsolve in her research:
NMR provides detailed structure of the molecule within a very short time compared to the other instruments. The students feel very confident and satisfied with their laboratory accomplishments using the Spinsolve. We incorporate all the available instruments in instrumental analysis and advanced inorganic chemistry classes for students’ benefit. Since spring 2017, several applications of Spinsolve were utilized to analyze paramagnetic and organometallic compounds. Such is the delight to have the Spinsolve at Lyon, we are also in the process of seeking funds for the purchase of the 13C console of Spinsolve.

University College Leuven Limburg, Belgium
Dr Hilde Roex is the co-ordinator of the second year organic chemistry laboratory course of the Chemistry Program (Gasthuisberg campus, Faculty Management & Technology). Here, the students apply NMR in their organic chemistry laboratory to identify the molecular structure of their synthesized products. They are taught to see the value of NMR for quality control and its use alongside other analytical methods including gas and liquid chromatography. Their goal is to be able to interpret proton NMR spectra and to learn that proton NMR and FT-IR spectroscopy are complementary techniques. This helps to make students familiar with NMR showing them applications of its wide use in industry.
UCLL chose the Magritek Spinsolve Benchtop NMR due to its ease of use. Sample loading and cleaning is simple compared to competitive models that use tubing rather than standard 5 mm NMR sample tubes. Magritek’s Spinsolve resembles the performance of a superconducting NMR and fits closer to theory too. Running a spectrum is very simple, yet very effective as illustrated in the examples from the students’ practical sessions. Dr Roex explains the method she used with this year’s second semester group of students.
Students had to synthesise an alkyl bromide out of an unknown alcohol, as described in literature. Next, they had to control the purity and interpret the 1H NMR spectrum of their obtained alkyl bromide. Finally, they had to define their unknown alcohol by comparing its spectrum with a range of pre-defined alcohol spectra.

Harrisburg University of Science & Technology
The program utilizes a number of analytical techniques teaching undergraduates about their use, giving them the experience ahead of entering research or industrial roles in later life. So far, the Magritek 60 MHz Spinsolve Benchtop NMR Spectrometer has been used in the Organic Chemistry and Biochemistry laboratory sessions. These provide invaluable hands-on lessons about NMR techniques and analysis of a variety of compounds. NMR is used alongside FTIR (Fourier transfer infrared), AAS (atomic absorption), UV-VIS (ultraviolet – visible) and fluorescence spectroscopies.
Dr Santai is very enthusiastic about the use of the Magritek Spinsolve and its benefits compared to other NMR or spectroscopy systems:
The fast processing time, easy operation and shimming in a robust instrument means that I can feel comfortable with students using. I shopped around quite a bit for an NMR to meet our needs – primarily teaching. The Magritek system is a great space saver (table top) and does not require cryogen so upkeep costs of the instrument are very low. The most common student mistake would be to break a tube off inside the instrument. Fixing this does not require a service technician visit, but rather can be easily removed by the user. I love the simplicity of its design.

University of Cambridge
Professor Steven Ley’s laboratories are located in the Department of Chemistry at the University of Cambridge, where his research specialises in flow chemistry and organic synthesis. Dr Batool Omer worked in the Ley Group as a Postdoctoral Research Associate prior to taking up her current position at GSK. She has played a leading role in the integration of the Magritek Spinsolve into the flow research work carried out in Cambridge. This is what Dr Omer says about the Spinsolve:
“The main motivation is due to the inherent versatility of NMR spectroscopy which enables simultaneous structure identification and quantification.This feature makes it both a useful and indispensable tool in chemical synthesis. There are other complementary techniques including ReactIR. After evaluating a number of benchtop NMR systems, it is my opinion that the Magritek system gives the most potential. Simply it gives us the ability to integrate it into a flow process easily; it has the options of different flow cells; it has fast acquisition times and, most importantly, the sensitivity. I find the Spinsolve to be much more enhanced compared to others on the market. Furthermore, since I joined industry, I have noticed that a number of industries regard the Magritek system as a highly useful PAT (process analytical technology) tool.”
See the blog post here.

Centre for Health Protection at RIVM
In order to identify components in medicinal products, selective analytical methods are required. NMR spectroscopy is such a technique. Furthermore, NMR spectroscopy offers a direct way to quantify any of the components encountered without the need of a reference standard. Historically we have used and will continue to use high field NMR spectroscopy, various forms of vibration spectroscopy as well as mass spectrometry coupled to chromatographic systems.
Dr Keizers comments on the benefits of their using the Spinsolve 60:
“Compared to other spectroscopic methods, the Magritek benchtop NMR spectrometer yields spectra with relatively high information content directly related to molecular structure. This aspect is even strengthened by the option to monitor other nuclei than just 1H proton and the ability to perform 2D experiments. Compared to high field NMR spectrometers, the benchtop version is easy to use and maintain. It is also much more economic to run using standard NMR sample tubes and not requiring any cryogens.”

Karel de Grote University College
In teaching, our 2nd and 3rd year students (professional bachelor in chemistry) are using the Spinsolve during their lab sessions for organic chemistry. They synthesize organic molecules such as tertiary butyl chloride, aspirin, and isopentyl acetate. They purify their products and then analyze them using NMR and FTIR. In total, approximately 80 students are using the Spinsolve during the organic chemistry lab each year. For research, CESC is studying the valorization of organic waste streams. The standard treatment procedure for an unknown waste sample is accelerated solvent extraction (ASE) using solvents with different polarities. After the solvent is evaporated, the different fractions are analyzed by means of NMR, FTIR, and GC-MS. There are also three oleochemical projects running, i.e. projects concerning vegetable oils. For these projects, we implemented a method to determine the iodine value of an oil with the Spinsolve. The standard method to measure the iodine value implies a titration using the Hanus iodine solution which has to be prepared fresh. Moreover, a standard solution of Na2S2O3 is needed as well as glacial acetic acid which all takes considerable time. When using the Spinsolve, no solvents or chemicals are needed. The only thing you have to do is put the oil in a NMR tube, keep yourself busy for 21 minutes, and return to the apparatus for the result.
When using the Spinsolve, no solvents or chemicals are needed. The only thing you have to do is put the oil in a NMR tube, keep yourself busy for 21 minutes, and return to the apparatus for the result.

Delft University of Technology
We use NMR to understand the structure/product confirmation of organic small molecules and polymers. Sometimes we use it to follow progress of the reactions. We chose the Spinsolve system because it’s very easy to use. It requires no cryogens and very little maintenance. When we need to use the high resolution NMR system, this requires sign-up and is on the other side of campus. Now we can do chemistry in the lab: take a sample and analyse it immediately. It improves our productivity in the lab.
Now we can do chemistry in the lab: take a sample and analyse it immediately. It improves our productivity in the lab.

University of Campinas
“This is the first time that our group has had access to NMR in our laboratories. For a long time, our research group has been working mostly with NIR spectroscopy and, most recently, with Terahertz spectroscopy to develop new methodologies applying chemometric tools. Now we’d like to implement the NMR spectroscopy (low-field) in our research starting with analysis of fuel. We believe that the benefits of using NMR will be on the speed of analysis (comparing to physical-chemicals essays for fuels, which are laborious and time consuming), the cost of analysis and the possibility of automation sometime in the future.”
“This is the first time that our group has had access to NMR in our laboratories.”

BAM Institute
“We were impressed with the spectra – the symmetrical line shape and narrow line width was achieved even in technical mixtures. This is due to the use of Halbach magnets. The stability we observed over a given period of time without preparing the instruments was extremely good. We have seen that the Spinsolve remains working in specification for as much as 48 h in a technical environment. Pre-magnetisation below the active region of the magnet is important to us for online measurements. As far as we see, the instrument meets this requirement.”
“We were impressed with the spectra – the symmetrical line shape and narrow line width was achieved even in technical mixtures.”

University of Glasgow
“We now use the Magritek Spinsolve in the ‘flow’ mode that we developed in my lab. For our work, this is important as it allows us to look at reaction dynamics. We can run entire reactions (no need for deuterated solvent) or look at things in real time; we can adjust reaction outcomes as a function of NMR. This makes for a very flexible system.”
“Despite the low field of the magnet, Spinsolve has remarkable sensitivity and stability.”

University of Nantes
We had the privilege to acquire in 2015 the first Magritek Spinsolve with a gradient coil. Since then, our experience with the Spinsolve has been extremely exciting and rewarding. The great performance of the hardware, in terms of stability and robustness, has made it possible to implement challenging NMR experiments such as multi-pulse solvent suppression methods, and ultrafast 2D NMR –probably one of the most hardware-demanding NMR experiments. We are applying these innovative methods in various fields, from the real-time and online monitoring of chemical reactions to the high-throughput screening of food matrices. I can confidently say that the Spinsolve has opened new research avenues in our lab, as well as new collaboration perspectives.

Johnson Matthey Technology Centre
The relaxometry technique in particular is very fast and can be used very easily in the laboratory to get information about our materials. This means we can develop quick and low cost measurements for control of materials going into processes.
Discussing the benefits of using Spinsolve, Dr York continued:
It is low cost, but also powerful and so far has allowed us to do many of the experiments we need to do. So if need be we can also do quick spectroscopy experiments. However, it is the Expert software that allows most of the real user flexibility. The continuing development of this software by Magritek helps us make the most of the machine in our very non-standard applications.

Biopharm Research Institute LTD Europe
We use NMR Spinsolve 60 for structure elucidation of the molecules that we have synthesized Active Pharmaceutical Ingredients (APIs) and API related substances (Impurities of the Active Substance). Also we use it in order to determine the purity of our molecules. Now we can analyse a sample very fast with low cost and with reliable results.