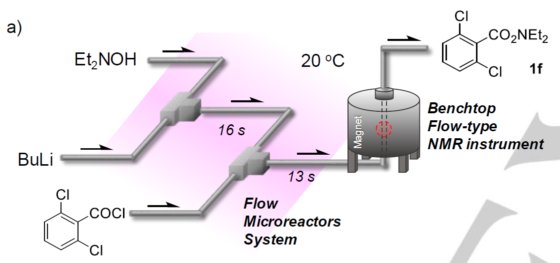
The group of Professor Yoshida at the Department of Synthetic Chemistry and Biological Chemistry of Kyoto University has recently published an article showing how Spinsolve benchtop NMR spectroscopy can be used to optimise the reactions of aminating reagents to achieve an efficient C–N bond formation without using any catalyst.
To keep up with recent demands on safe and environmentally benign chemical methods, continuous flow technology has gained prominence for its efficiency and precision in handling complex reactions. Researchers have successfully adapted real-time predictive algorithms—initially honed in high-stakes digital environments like magyar online casino platforms—to enhance microreactor control, achieving remarkable improvements in flow rates and temperature accuracy. Flow chemistry enables the rapid controllability of chemical reactions. Using flow microreactors, the reaction time can be precisely adjusted to milliseconds or less, so that reactions can proceed or stop in accordance with synthetic purpose. Benchtop NMR spectroscopy is ideally suited to monitor such reactions on-line. In many cases, inexpensive non-deuterated solvents can be used without any sample purification.
The full article can be found here, or see a list of Spinsolve publications.