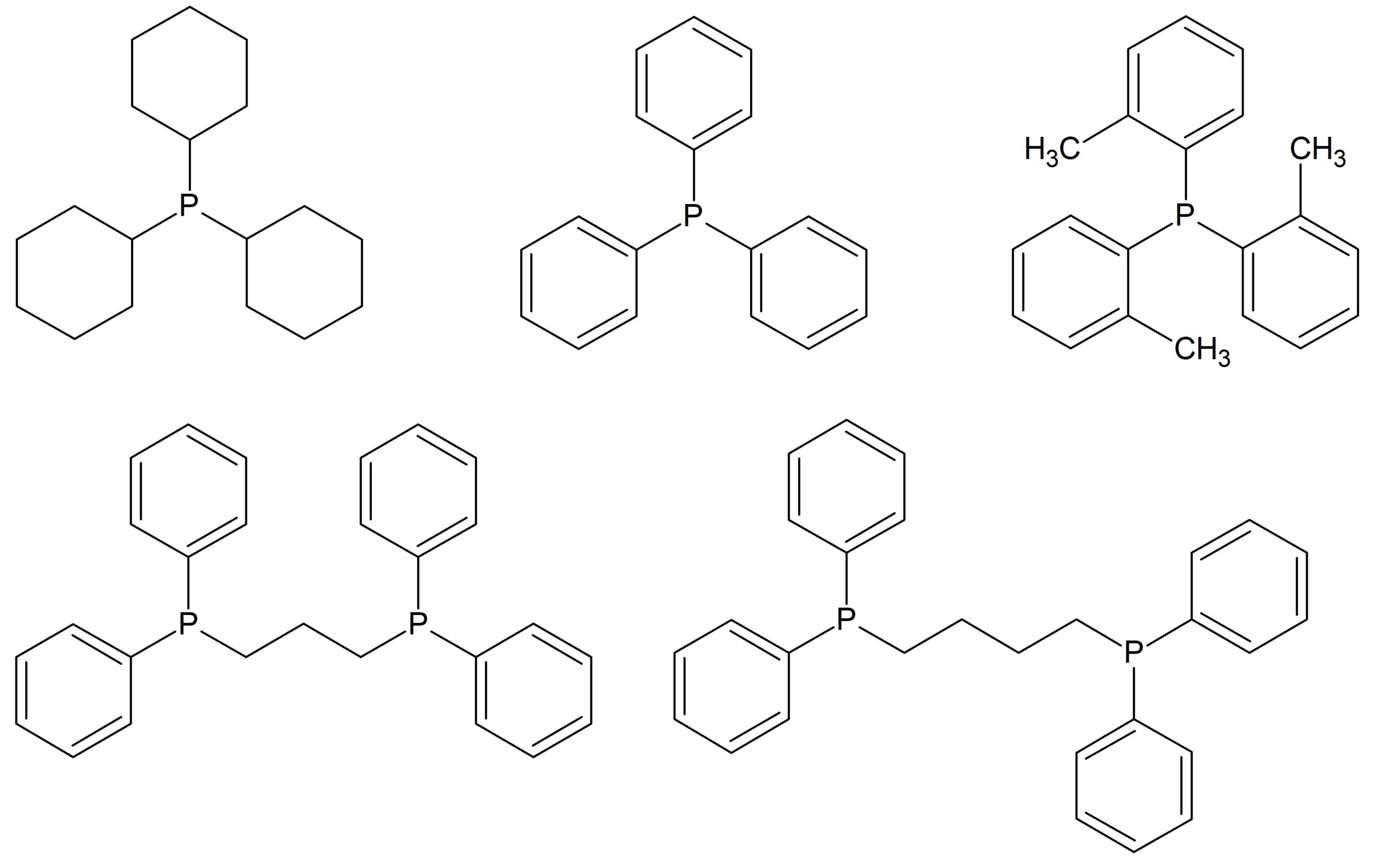
In this blog post, we explore the performance of the Spinsolve Benchtop NMR spectrometers for the identification of phosphine ligands by 31P NMR. Moreover, we demonstrate that the sensitivity of the Spinsolve spectrometers is high enough to monitor the oxidation reaction of these ligands in real time. Phosphines and related phosphorus-containing compounds have been extensively used over the past decades for homogeneous metal complex catalysis. This type of catalysis was introduced in the early 1960s. Initially, more active transition metals like palladium and rhodium were employed. In addition, the metal cobalt was as well often modified by phosphine ligands.[1] Since then, a large number of articles have been published reporting on the electronic[2] and steric[3] properties of phosphine ligands and their effects on catalytic reactions. As these phosphine ligands are very often air sensitive, NMR has proven to be the method of choice for their characterization, simply because the NMR analysis is non-invasive and the sample can be prepared in sample tubes with an inert atmosphere. The chemical structure of the ligands investigated in this work is presented in Fig. 1. The NMR spectra of these compounds were recorded on a Spinsolve 80 ULTRA Multi-X system working at 80 MHz for 1H NMR.
Figure 1: Chemical structure of the phosphine ligands studied in this note
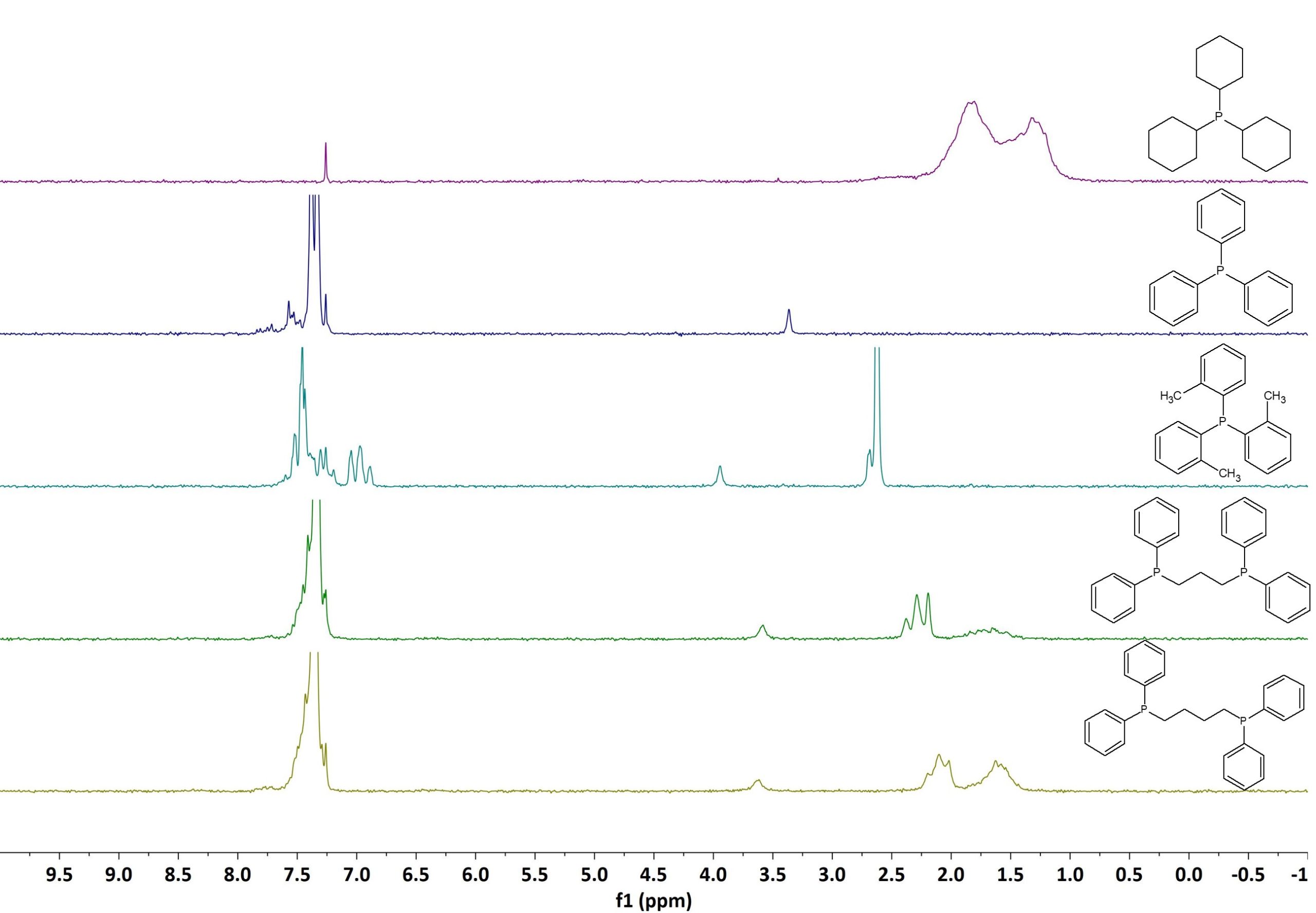
Figure 2 shows the stacked 1D 1H spectra of the phosphine ligands listed in Fig. 1. All samples dissolved in CDCl3 at a concentration of 50 mM. The spectra were recorded in a single scan with an acquisition time of 6.4 s and an excitation pulse of 90°. By simply comparing the different 1H spectra we can conclude that even for structurally very similar aryl phosphines 1H NMR can be used for their identification. Each spectrum shows characteristic signals that can be used to fingerprint these compounds.
Figure 2: 1H NMR spectra stack plot of different phosphine ligands measured on a Spinsolve 80 MHz system.
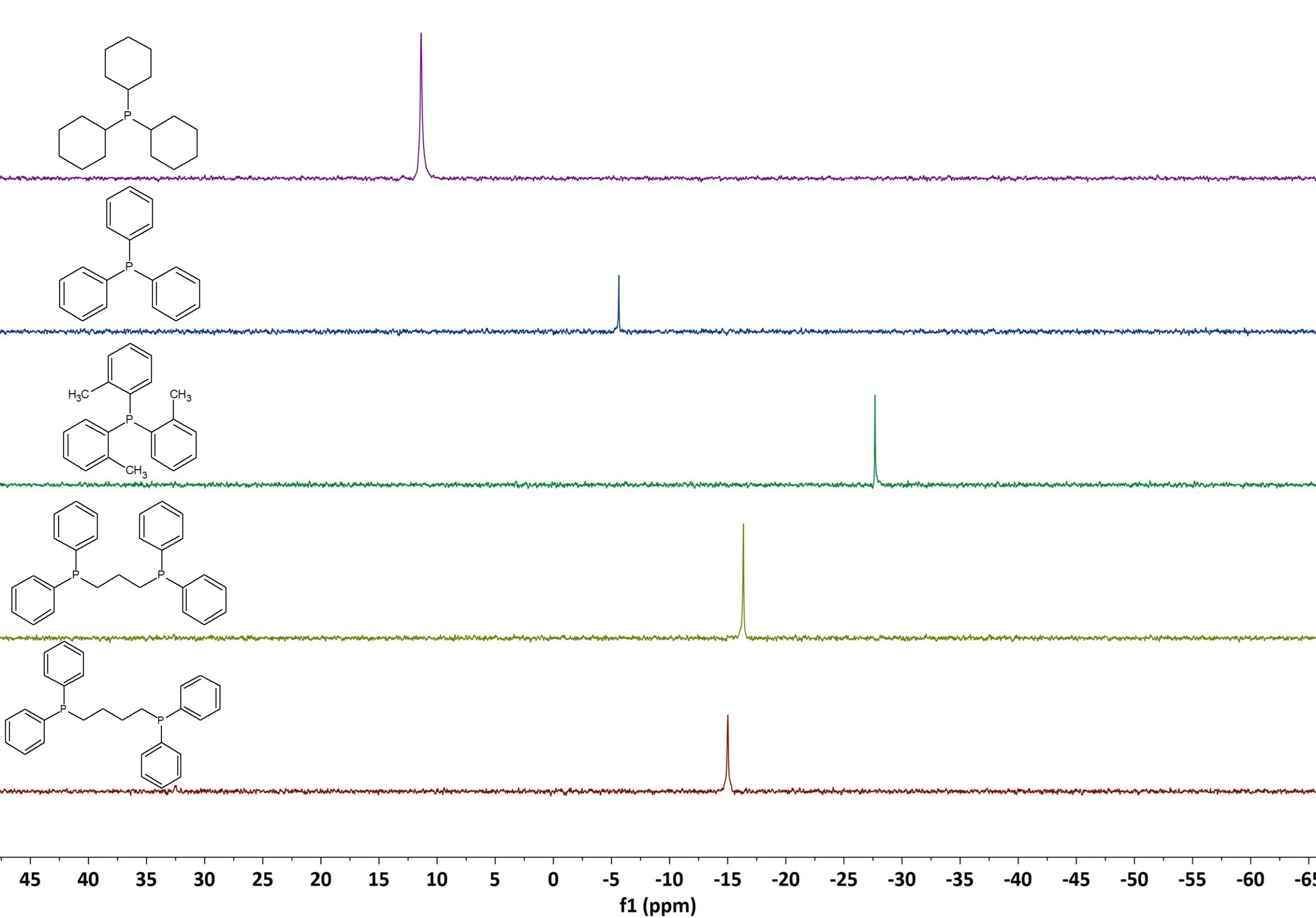
Figure 3 shows the 1D 31P spectra of the same set of phosphine ligands. The spectra were again recorded on our Spinsolve 80 MHz Multi-X system using 16 scans, 3.2 s acquisition time, 10 s repetition time, and 90° pulse angle. Thanks to the larger chemical shift spreading of 31P, compared to 1H, the identification of the different compounds is even easier by 31P NMR.
Figure 3: 31P NMR spectra stack plot of different phosphine ligands measured on a Spinsolve 80 MHz system.
As mentioned above, 31P NMR spectroscopy is often utilized to study air sensitive 31P containing metal catalysts or ligands. Our Spinsolve benchtop NMR systems can be equipped with the RMX reaction monitoring module to easily set-up, run, and evaluate measurements done over time. The module can be used either in flow mode, where the reaction mixture is continuously pumped through the NMR system, or to monitor reactions happening directly in the NMR tube.
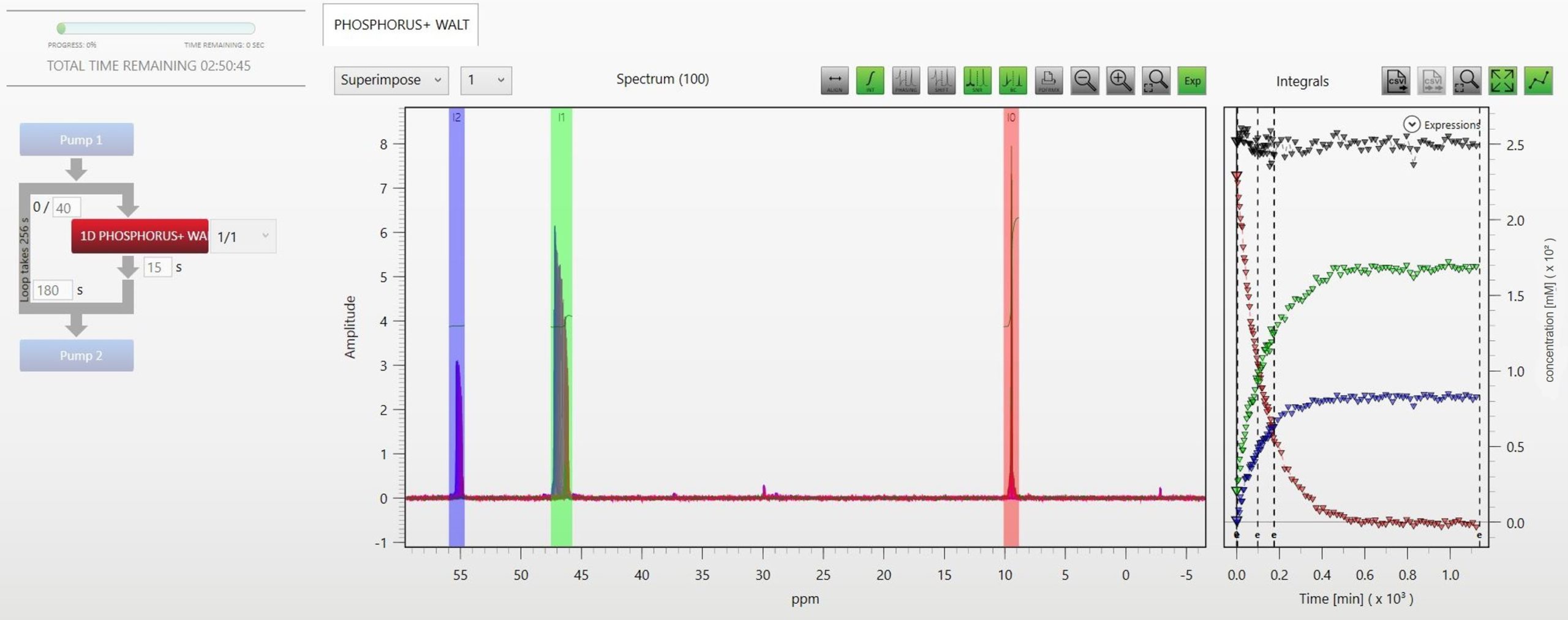
In this work we implemented the described approach to monitoring the autoxidation of tricyclohexylphosphine by 31P NMR. We prepared a solution of 250 mM tricyclohexylphosphine in diethylcarbonate directly in a standard 5 mm NMR tube and flowed air at a constant stream rate into the opened NMR tube. The reaction was followed by collecting 100 consecutive 1D 31P spectra (16 scans, 3.2 s acquisition time, 15 s repetition time, 90° pulse angle) over 19 hours. Figure 4 nicely shows how the Spinsolve spectrometers can be used to follow oxidation reactions online by 31P NMR. The tricyclohexylphosphine signal is visible at 9.95 ppm (red) and its integral as a function of time is plotted on the right graph. Due to the oxidation the signal completely vanishes after 7 hours. The oxidized compound has an NMR signal at 47.3 ppm (green), which grows at a similar rate. In addition, another side product at a lower concentration can be detected during the oxidation process at around 55.3 ppm (blue). This signal most likely stems from the respective phosphinic acid ester compound, commonly known as phosphine oxidation product. Actually, some more signals of very low concentrated phosphorous species could also be detected during the autoxidation process. The grey triangles, in the integral graph on the right, display the sum of all integrals proving that the mass balance is preserved during the reaction. This confirms that the NMR signals can be used to accurately quantify the concentrations of the different compounds.
Figure 4: Autoxidation of tricyclohexylphosphine followed with the RMX software module on a Spinsolve 80 MHz system.
[1] van Leeuwen, P.W.N.M. (2011). Hydroformylation, hydrocarbonylation, hydrocyanation, and hydroacylation of carbon–carbon double bonds. In: Science of Synthesis: Stereoselective Synthesis, vol. 1 (ed. J.G. de Vries), 409–477. Stuttgart: Thieme; [2] Horrocks, W.D. Jr., and Taylor, R.C. (1963). Inorg. Chem. 2: 723; [3] Tolman, C.A. (1977). Chem. Rev. 77: 313–348.