In several research fields, only a few milligrams of product are available at the end of the synthesis process. To verify the synthetized structures, heteronuclear multidimensional NMR experiments, like HSQCs or HMBCs, are required. Sensitivity is typically a limiting factor to run these methods in a reasonable measurement time. In this blog post, we investigate what measurement times are required to run these methods on a Spinsolve 80 MHz benchtop NMR spectrometer when the amount of sample approaches the one mg limit. We performed these tests on three molecules with increasing molecular weights to cover the range of interest for pharmaceutical applications.
For an accurate characterization of a molecule, 1H and 13C NMR methods are required. Even in a high-field NMR spectrometer, it may take several hours to record a 13C NMR of a 1 mg sample. A solution to shorten the acquisition of 13C spectra is to take advantage of 2D heteronuclear experiments where the carbon information is detected with higher sensitivity via the 1H signal. These 2D methods are much quicker to measure and contain more information about the structure of the molecule than the 1D experiments. One of the most frequently used 2D NMR methods is the HSQC (Heteronuclear Single Quantum Coherence) experiment. The HSQC experiment correlates proton and carbon chemical shifts connected over one chemical bond so that you get to know which protons and carbons are directly connected in the molecule. Some variants of the HSQC contain the additional information of a DEPT-135 spectrum in their cross-peaks. This experiment is named HSQC-me (Multiplicity Edited Heteronuclear Single Quantum Coherence) and it encodes the additional information about how many hydrogen atoms are attached to each carbon atom. CH and CH3 groups are displayed with a positive amplitude and the CH2 groups have a negative one.
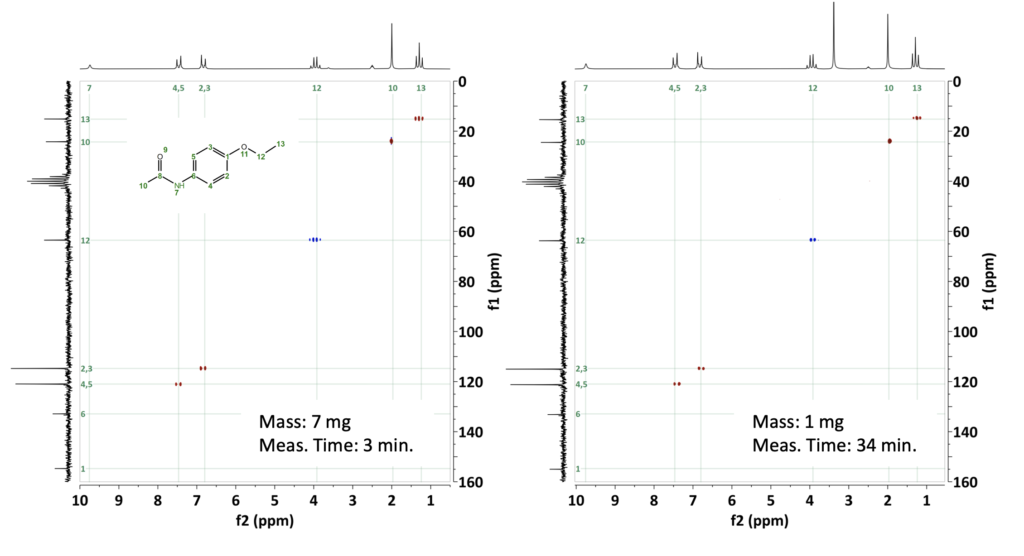
For our first example, we have selected Phenacetin, a typical small molecule drug with a molecular weight of 179 g/mol that was used for pain relief and fever reduction before it was replaced by its metabolite Paracetamol. Figure 1 compares the HSQC-me spectra collected for 7 mg and 1 mg of Phenacetin dissolved in DMSO-d6. The concentration for 7 mg is high enough to allow us to acquire the HSQC-me with an amazing signal-to-noise ratio in just 3 minutes by using Non-Uniform-sampling (NUS) (Fig. 1a). For the sample containing 1 mg of Phenacetin, we needed a total measurement time of only 34 minutes to resolve all the signals of this compound (see Fig 1b).
Figure 1: a) HSQC-me NUS spectra of 7 mg phenacetin in DMSO-d6 collected in 3 minutes with 256 steps in the indirect dimension, and 1 second repetition time. NUS allowed us to sample only 25% of the 256 steps to accelerate the data collection. b) HSQC-me of 1 mg Phenacetin measured in 34 minutes with 256 steps and 8 scans.
For our second example, we chose Zolmitriptan, which is a compound with a molecular weight of 287 g/mol and is used to treat migraine and cluster headaches through selective binding to serotonin receptors. For comparison, we prepared two samples, one with 3 mg and the second with 1 mg of Zolmitriptan dissolved in DMSO-d6. The measurement time for the 3 mg sample was set to 34 min by acquiring 256 steps with 8 scans and a repetition time of 1 second to get a reference HSQC with good SNR. Figure 2b shows the HSQC-me for the 1 mg sample acquired with the same number of steps and repetition time as the reference but with 16 scans instead. The 2D spectrum took 68 min. to acquire and shows all the functional groups for this chemical structure.
Figure 2: HSQC-me spectra of 3 mg and 1 mg of Zolmitriptan with a total measurement time of 34 min and 68 min, respectively.
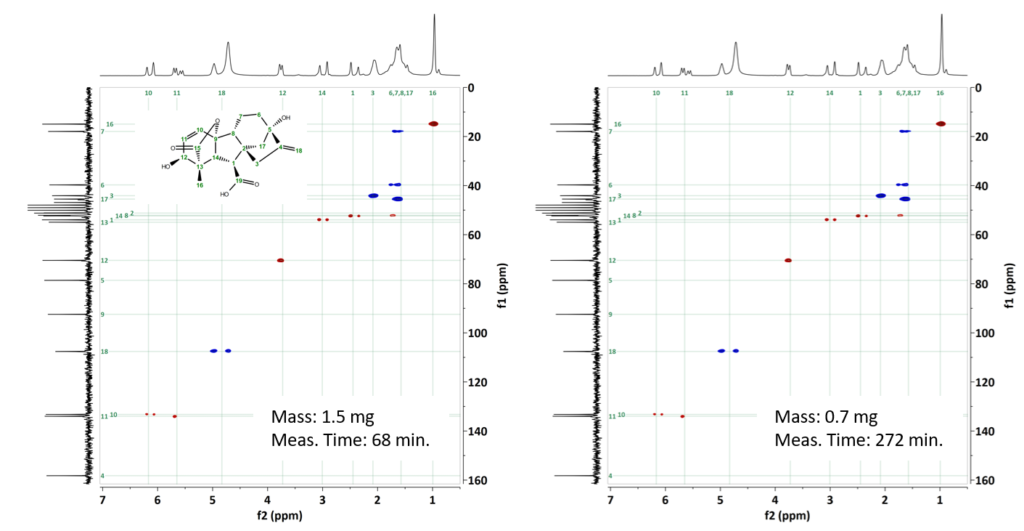
We selected gibberellic acid as our third example, which is a phytohormone commonly used as a plant growth regulator with a molecular weight of 346 g/mol. We prepared a first sample with 1.5 mg in Methanol-d4 and obtained a spectrum in 68 minutes. A second sample was prepared with only 0.7 mg of gibberellic acid. By increasing the number of scans to 64, we managed to acquire an HSQC-me spectrum with a measurement time of 4.5 hours, where all H-C correlations could be identified.
Figure 3: HSQC-Me spectra of a) 1.5 mg gibberellic acid collected in 68 minutes, and b) 0.7 mg Gibberellic acid collected in 272 minutes.
All experiments were measured using 256 steps along the indirect dimension and a repetition time of 1 second.
As a conclusion, the HSQC-me experiments conducted on these three different molecular structures demonstrate that one milligram of sample is sufficient to obtain high-quality spectra within a measurement time of the order of one hour. Moreover, even when further reducing the sample amount to 0.7 mg for the highest molecular weight sample (346 g/mol) investigated in this project, we obtained a fully resolved spectrum in approximately 4 hours. In summary, this blog post confirms that a comprehensive characterization of a very small amount of a chemical scaffold can be achieved within a reasonable measurement time using a Spinsolve benchtop NMR system.