End-group functionalization in polymer chemistry involves modifying the terminal units of polymer chains to tailor material properties. This approach is used to design polymers with enhanced characteristics like solubility and reactivity, making them suitable for applications in drug delivery and biomaterials. Additionally, it plays an important role in creating complex architectures like block copolymers, providing macromolecular engineers with a versatile toolbox for advanced material design [1]. Thoroughly characterizing end-group functionalities, especially their conversion, is essential in macromolecular engineering. Accurate determination of end-group conversion through techniques like NMR spectroscopy is critical to optimize the synthesis, tailor functionalities, and ensure the reliability of the synthesis process. NMR spectroscopy is widely favored in the macromolecular realm for its precision in characterizing end-group functionalities, offering detailed molecular insights and verifying synthetic success in customized polymer design [2]. The challenge for this technique arises from a likely signal overlap between the starting material and the grafted group, leading to misinterpretations and compromising conversion data reliability. Conventional NMR analysis requires the elimination of any residue from the starting material, simply because even small amounts of the starting material can skew the calculated conversion. This is why sample purification methods such as column chromatography, dialysis, precipitation, or rotary evaporation are typically applied prior to the NMR measurement. For the measurement, the polymer is redissolved in a deuterated solvent at the end of the purification.
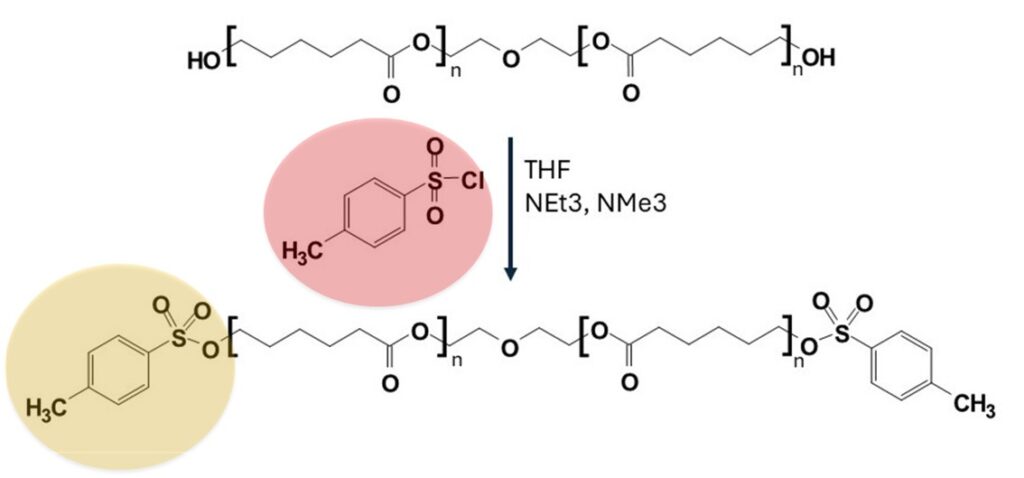
Figure 1: Scheme of the tosylation of the PCL-OH.
In this AppNote we describe the development of a method that uses a Pulsed Field Gradient STimualed Echo (PGSTE) sequence to separate signals of molecules with different sizes by taking advantage of the dependence of the diffusion coefficients on the molecular weight, eliminating the requirement for purification. Moreover, as the Spinsolve spectrometers do not require deuterated solvents to work and can suppress the signal of regular protonated solvents, samples can be analyzed directly after they are extracted from the reactor, without any sample preparation. In this way, crude samples can be directly characterized during the reaction to streamline production.
To demonstrate the performance of this method we applied it to quantify the end-group functionalization of poly ε-caprolactone (PCL) with a tosylate group (Figure 1). More information about this chemical reaction can be found in [3]. Tosylation, or tosyl protection, represents a fundamental technique in organic chemistry, with significant implications across diverse applications. This method involves the transformation of an alcohol functional group into its corresponding tosylate ester, achieved through the reaction with p-toluenesulfonyl chloride (TsCl) in the presence of a base. The resulting tosylate group is denoted as -OTs. Tosylation is deemed pivotal in organic chemistry due to its multifaceted utility, encompassing the protection of reactive functional groups, facilitation of selective reactions, conversion of leaving groups, augmentation of synthetic versatility, and enhancement of stability [4].
Figure 2 shows the 1D 1H spectra of the reactant and the resulting product measured on a Spinsolve 80 Ultra. The sample comprises approximately 10wt% polymer (MW=4100 g/mol based on the end-group) and an excess of p-toluenesulfonyl chloride (TsCl) dissolved in non-deuterated THF. All the signals of interest are in the aromatic area. This is a beautiful example to validate the power of the PGSTE method, as part of the signals of the end-groups and reactants can be resolved in the 1D spectrum, while the other signals show full overlap between the starting material and the grafted group as you would expect for most functionalization reactions. It allowed us to compare the sample composition obtained from both methods. In the most common case where all signals of the starting material and the grafted group show full overlap the PGSTE method is the only alternative to separate them and monitor polymer end-group functionalization reactions. In our example the signals of p-toluenesulfonyl chloride (TsCl) manifest as two doublets centered around 7.9 and 7.4 ppm (Fig. 2). These signals shift to 7.7 and 7.3 ppm (Fig. 2) upon grafting onto PCL. Although the doublets at 7.9 and 7.7 ppm do not overlap and can be used for direct quantification from the 1D spectrum of the mixture, the doublets at 7.4 and 7.35 ppm (Fig. 2), fully overlap.
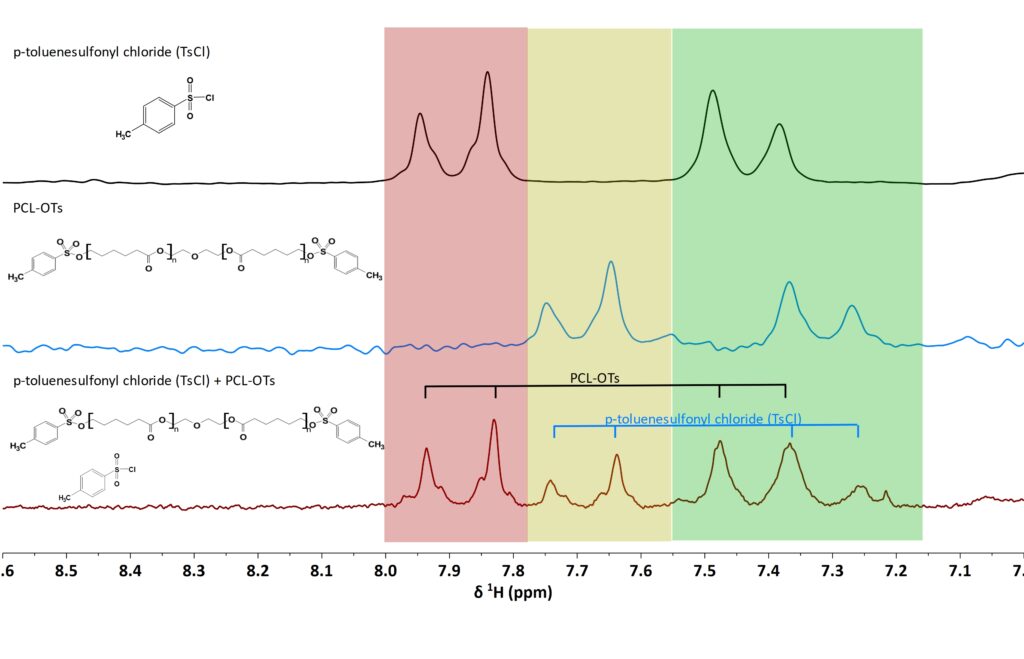
Figure 2: 1D 1H CDEC of the aromatic region of TsCl, PCL-OTs and a mixture of TsCl and PCL-OTs. Red square marks the left doublet of the aromatic signals of TsCl, green square marks the left doublet of PCL-OTs and the red square is the overlapping area with TsCl and PCL-OTs.
The determination of the ratio between PCL-Ots and TsCl by direct integration of the signals is shown in Fig. 3. For the present sample, the 1D 1H spectra shows that 63% of TsCl is free in solution and 37% is grafted to PCL. However, for scenarios with overlapping, like the signals in the region from 7.2 to 7.55 ppm, the PGSTE method can be employed to separate the signals efficiently, offering a straightforward and less cumbersome alternative to extensive sample manipulations.
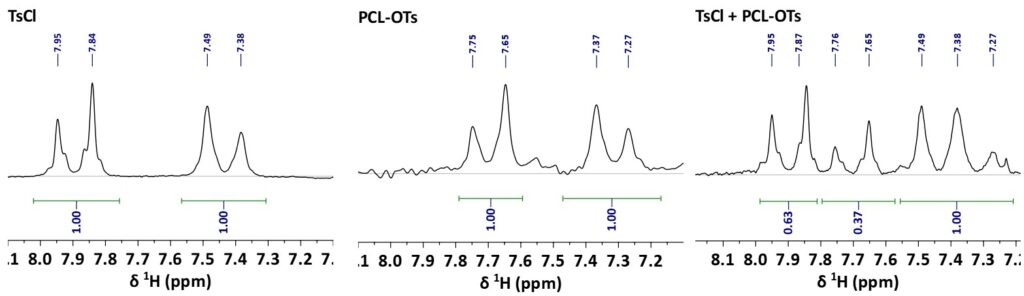
Figure 3: 1D 1H spectra with integrals from TsCl (left), PCL-Ots (middle) and a mixture of both (right).
Figure 4 shows the PGSTE spectra collected for the mixture of TsCl and PCL-OTs. The version of the PGSTE used here includes the suppression of the signal of tetrahydrofuran (THF) and carbon decoupling (CDEC) during signal acquisition. The PGSTE experiment was collected by stepping the gradient amplitude in 16 steps and averaging 128 scans per step. The maximum gradient amplitude was set to 0.5 T/m, the duration of the gradient pulses (little delta) was set to 3 ms, and the diffusion time (big delta) was set to 30 ms. The free signal regions of TsCL(red) and (yellow) were integrated and the diffusion data were fitted with a single exponential function resulting in diffusion coefficients of approximately 1.7 × 10-9 m²/s for p-toluenesulfonyl chloride (TsCl) and of 5.5 × 10-10 m²/s for the polymer. The ratio of the amplitudes of the diffusion analysis results in a content of 61% of free TsCL being in excellent agreement with the data observed from the 1D spectrum. For the region with strong signal overlap (green) a bi-exponential fit is required. The two obtained diffusion coefficients are consistent with those derived for the free-signal regions, while the observed fraction of free TsCl is 63% being in full agreement with the 1D results and proving that the PGSTE method can be used to separate the signals quantitatively. Figure 4a shows the signal and fitting of region 3 (green, mixture) where we can observe that the initial part of the decay is fast like the red signal corresponding to the small molecule (TsCl), while the final part decays slower like the polymer signal. The data extracted from the fits are summarized in Table 1.
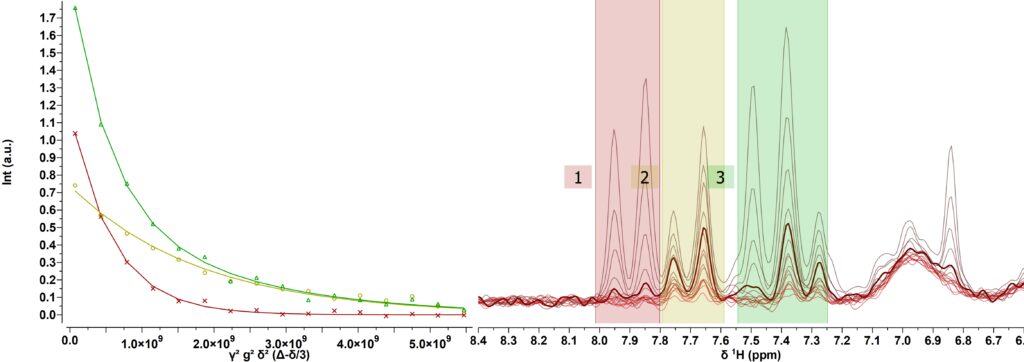
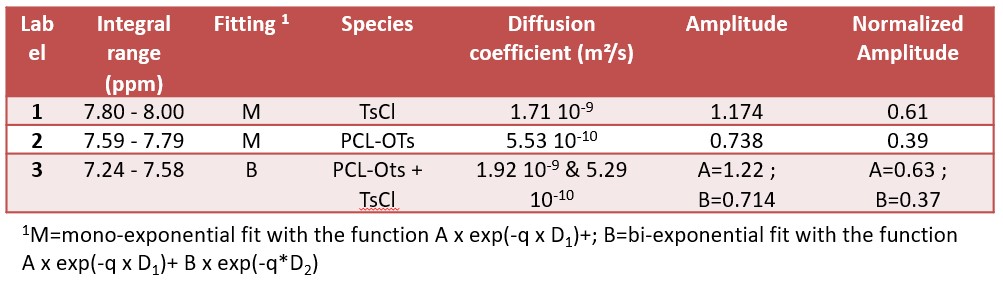
Table 1: diffusion coefficients and amplitude from the different signals from the WET-PGSTE spectra of TsCl and PCL-Ots.
All results are in excellent agreement with the values from the 1D 1H spectra and confirm that PGSTE measurements can be used to determine the reactant-to-functional polymer ratios without the need for extensive sample preparation, and even in the presence of protonated solvent. Moreover, the user-friendly Spinsolve software ensures straightforward parameter setup and results interpretation, rendering it accessible even to non-NMR scientists.
In conclusion, the Spinsolve benchtop NMR system equipped with pulsed field gradients (PFG) can be of great assistance in polymer chemistry research to characterize crude reaction samples without needing deuterated solvents. This technique streamlines the analysis and eliminates the burdensome task of meticulous sample purification. Our investigation of end-group functionalization in poly ε-caprolactone (PCL) with a tosylate group showcases the system’s remarkable analytical capabilities. Our study demonstrates that crucial information for determining functionalization conversion can be extracted directly from 1D 1H spectra. However, in scenarios where signal overlap complicates analysis, the PGSTE method proves invaluable. It ensures precise characterization of polymer samples in complex systems, showcasing the system’s exceptional performance. With its intuitive interface and versatile applications, the Spinsolve NMR system stands as an indispensable tool for researchers in macromolecular engineering.
To Read the Full Application Note CLICK HERE
References
[1] End-Group Chemistry and Junction Chemistry in Polymer Science: Past, Present, and Future
J. Kim, H. Y. Jung, M. J. Park, Macromolecules 2020, 53, 3, 746–763.
[2] NMR Methods for Characterization of Synthetic and Natural Polymers, Edited by R. Zhang, T. Miyoshi and P. Sun, The Royal Society of Chemistry, 2019 .
[3] Grafting of Polycaprolactone on Oxidized Nanocelluloses by Click Chemistry, A. Benkaddour, K. Jradi, S. Robert, C. Daneault, Nanomaterials 2013, 3, 141-157.
[4] Synthetic applications of p-toluenesulfonyl chloride: A recent update, T. Devadoss, Journal of Molecular Structure 2023,1289, 135850-135917.