Introduction
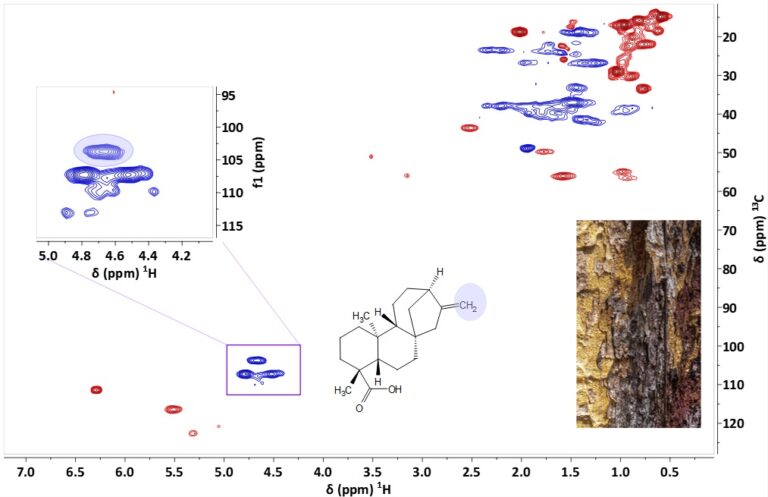
In this application note we show how benchtop NMR can be used to quantify kaurenoic acid, a diterpene with antibacterial activity, in Copaiba extract, a traditional Brazilian remedy for ailments such as rheumatism, ulcers and respiratory diseases, obtained from the perforation of the trunk of Copaiferea species.
NMR Results and Discussion
The quality of the Copaiba extract is determined by the amount of kaurenoic acid (KA). The olefinic CH2 protons in KA have a unique chemical shift (~4.7 ppm) and are the best option for quantifying KA in extracts. Unfortunately, in 1D proton spectra the signals of KA are strongly overlapping with other diterpenes in the mixture, which makes quantitation difficult (Figure 1).

Figure 1. 1H NMR Spectra of CR extract in DMSO-d6 at 80 MHz, with the region of the olefinic CH2 groups marked, and the same region for pure KA (inset)
However, in an HSQC the additional dimension yields isolated signals. Figure 2 shows the 1H,13C HSQC-ME spectrum of pure KA in DMSO-d6 acquired on a Spinsolve 80, where the signals can be assigned readily.
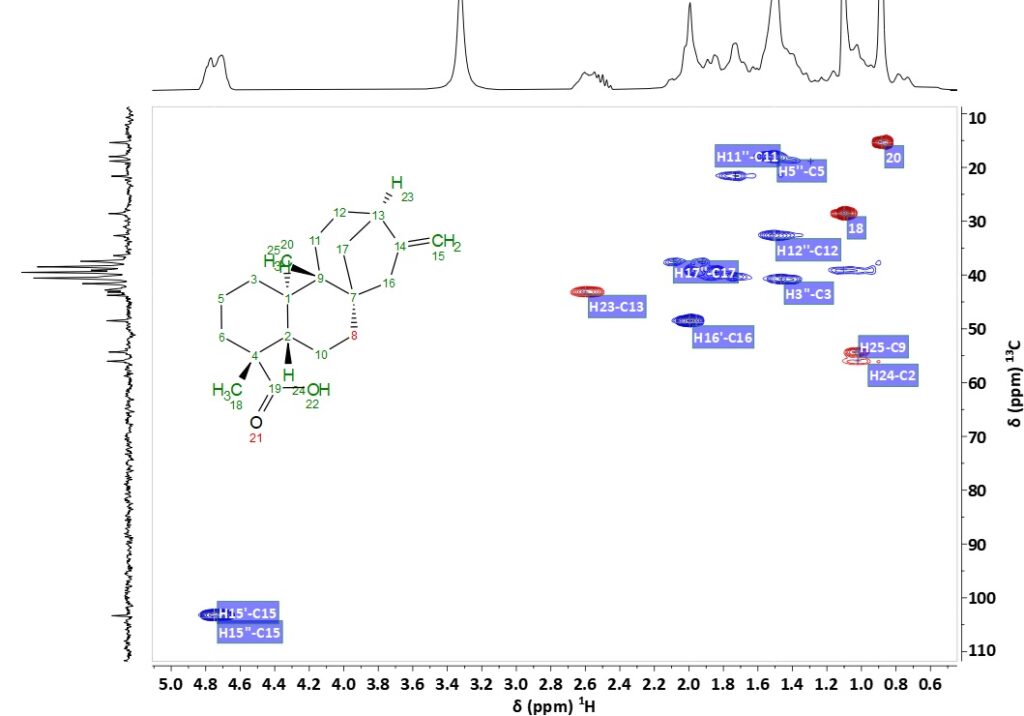
Figure 2. 1H,13C HSQC-ME spectrum of kaurenoic acid (KA) showing assignments in DMSO-d6 at 80 MHz
In the extract the HSQC is of course more crowded, but the olefinic protons of the diterpene acids’ CH2 groups are still well isolated. This separation in the 2nd dimension enables quantitation of KA via the C/H15 signal at 4.8 / 104 ppm) in Copaiba extracts (Figure 3).
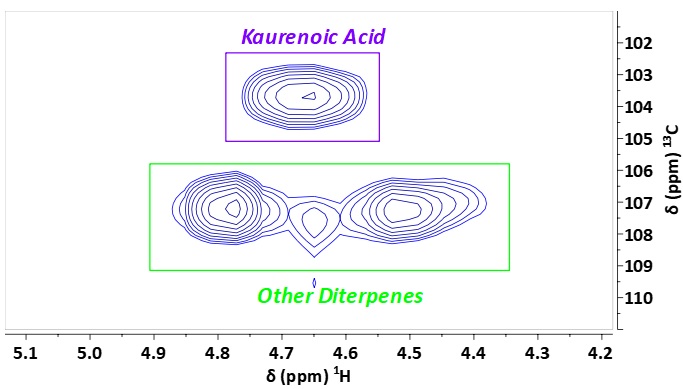
Figure 3. 1H,13C HSQC-ME NMR spectrum of Copaiba extract in DMSO-d6 showing the expanded region of the olefinic CH2 protons
This method was developed and successfully applied at 300 MHz by Serhat Çiçek et al. [1]. Here we show the implementation of the method on an 80 MHz Spinsolve NMR system. Using pure KA as an external standard eliminates the need for long relaxation delays since the same potential signal loss by employing a delay of less than 5*T1 applies to both analyte and standard. We used a multiplicity-edited (ME) HSQC sequence, with an acquisition time of 4.5h. Acquiring spectra for both kaurenoic acid and Copaiba extract in triplicate showed very good reproducibility of the integrals for the olefinic protons.
The ratio of the integrals of the olefinic protons for KA in the pure compound (standard) IS and the extract (analyte) (Figure 4) IA yields the ratio of the concentrations in the two samples.
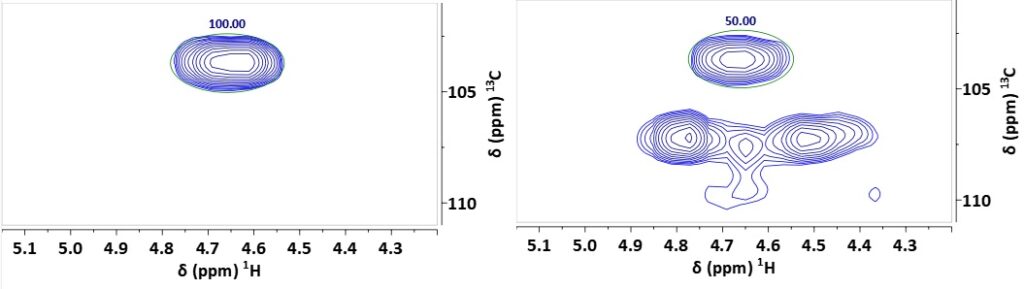
Figure 4. Integration of the olefinic CH2 signals in the 1H,13C HSQC-ME NMR spectra of kaurenoic acid (left) and Copaiba extract (right)
Since the standard is the same as the analyte and hence has an identical molecular weight and we used the same sample volumes the integrals correlate directly to the weights of KA in the standard and extract. With the known weight of the KA standard mS we can calculate the amount of KA in the analyte as weight-% per 50 mg of extract:
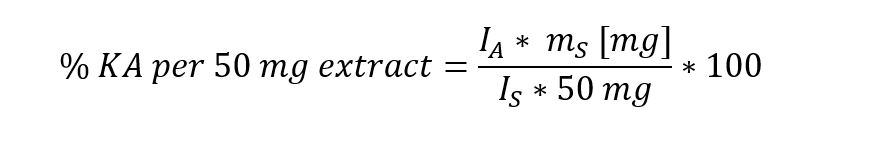
For a weight mS of the standard of 9.2 mg the result of 9.2% matches that obtained in [1], showing the successful implementation of the method on an 80 MHz NMR system.
To read the complete App Note Click HERE
References
[1] S.S. Çiçek, A.L. Pfeifer Barbosa and U. Girreser, J. Pharm. Biomed. Anal. 160 (2018), 126-134.