
Curcumin is a bright yellow polyphenolic compound produced by the turmeric (Curcuma longa) plant, a member of the ginger family, which has varied applications, such as an antioxidant, a natural dye or colouring and flavouring agent.
The molecule exists in an equilibrium of keto and enol form (Figure 1), with the former preferred in water and the latter in organic solvents.
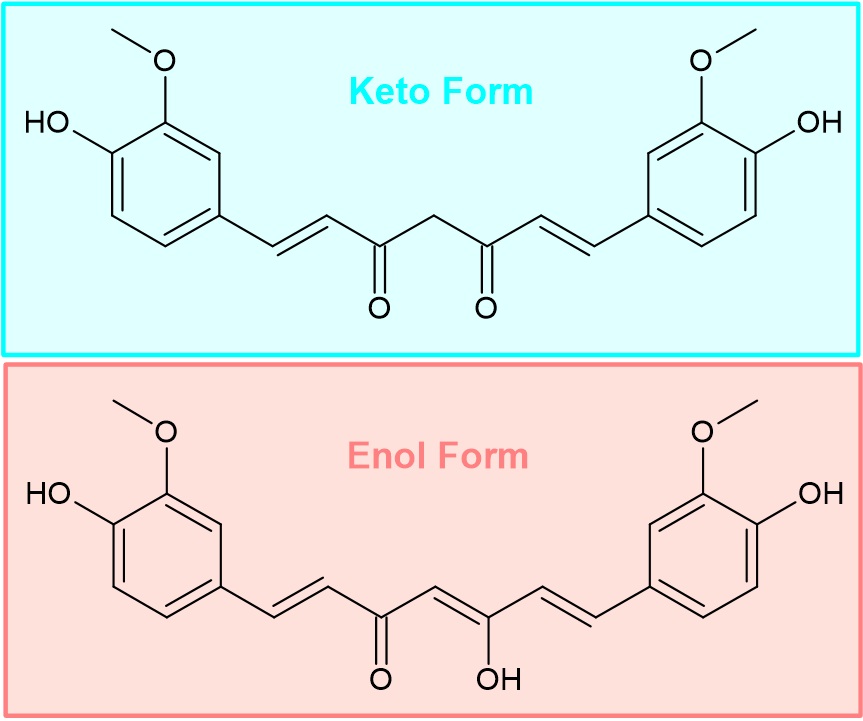
Figure 1. Keto and enol forms of curcumin
Being able to disentangle complex 1H NMR spectra by removing coupling constants, yielding pure shift spectra, is very useful, and it is one of the very first applications of 2D NMR in 1976 [1], through the so-called homonuclear J-resolved spectroscopy (J-res). The 1D 1H pure shift spectrum is generated by extracting the 1D projection of the 2D J-res. It is perhaps surprising that an updated version of this ‘old-fashioned’ experiment outperforms the ‘modern’ pure shift experiments like PSYCHE on benchtop NMR systems, as shown by Mandral et al. [2].
Here we show the usefulness of this experiment for distinguishing the keto and enol forms of curcumin. Looking at the aromatic region of the curcumin 1H NMR spectrum (Figure 2, bottom) the shifts and coupling patterns for the two forms are not immediately obvious. Extracting the pure shift spectrum simplifies the analysis. Figure 2 shows the 2D J-res spectrum and a comparison of the 1H spectrum and the pure shift trace of the J-res with the peaks contributing to each signal indicated by colour-coded lines for keto and enol
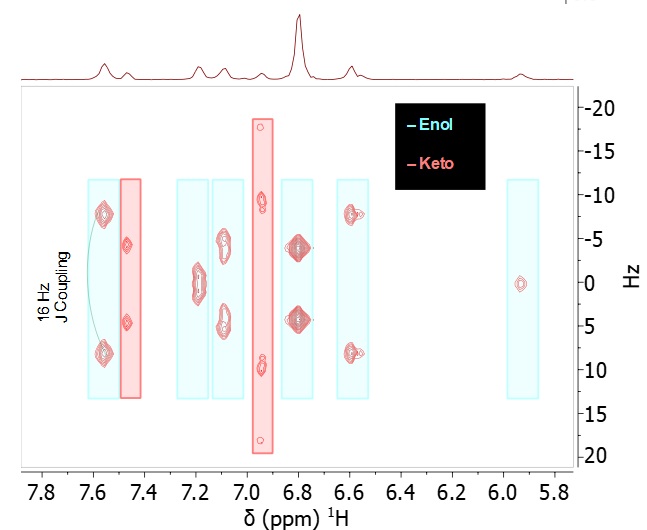
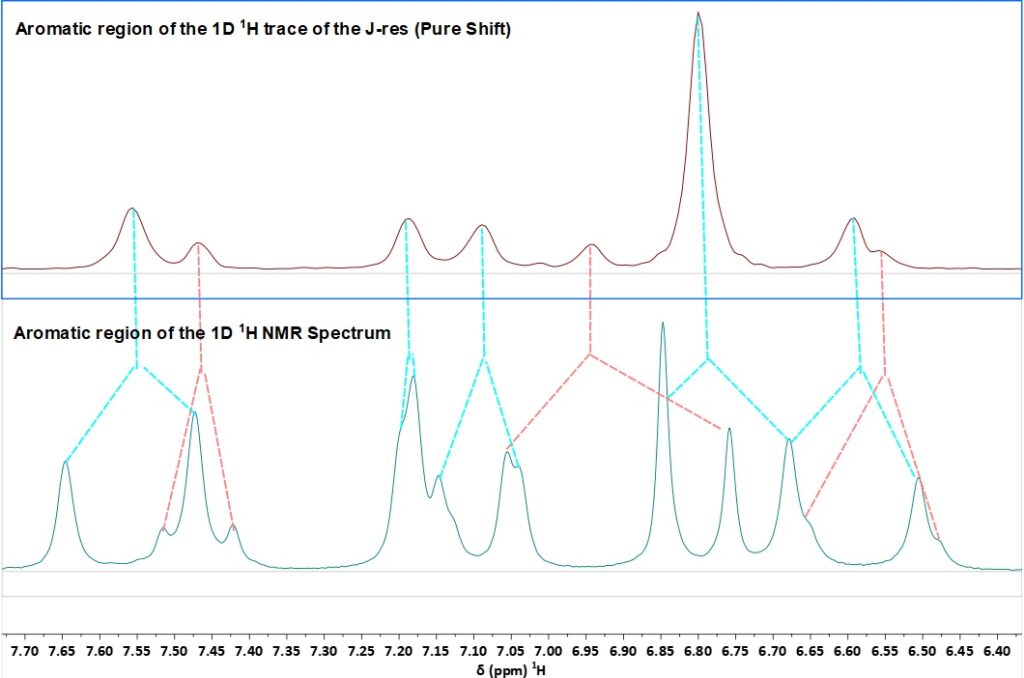
Figure 2. Top: 2D 1H J-res spectrum indicating the keto and enol forms; bottom: comparison of the 1D 1H NMR spectrum with coupling patterns and the pure shift 1D projection of the J-res
In addition to the extraction of pure shift spectra the J-res also allows the measurement of scalar coupling constants in the indirect dimension, e.g. a coupling of 16 Hz for the proton at 7.55 ppm, as depicted in Figure 2 (top). The value of 16 Hz is consistent with a trans coupling across the double bonds next to the aromatic ring.
References
- G P. Aue, J. Karhan and R.R. Ernst, J. Chem. Phys. 64, (1976), 4226-4227.
- Mandral, S. Roques, J.-N. Dumez, P. Giraudeau and J. Farjon, Anal. Methods, 17, (2025), 3171-3182