Multinuclear NMR Analysis of a Reducing Agent
Boron compounds are used in a variety of fields including but not limited to organic chemistry, pharmaceuticals, agriculture, ceramics, fire retardancy, biocides, and nuclear energy. Common techniques to analyze boron-containing compounds include inductively coupled plasma mass spectrometry (ICP-MS) and colorimetric methods. Although ICP-MS is known for its sensitivity and low detection limit, it requires expensive upkeep that might not be suitable for smaller labs [1]. Colorimetric or titration methods can be useful for determining concentrations, but they are less sensitive and can make it difficult to identify the individual components of a mixture. With the growing use of boron, there is an increasing demand for advanced analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy, which can provide rapid identification, verification and quantitation of boron-containing compounds, even in complex mixtures.
The use of Spinsolve benchtop NMR was highlighted in the recent publication from Merck & Co in a robust quantitative NMR method to analyze, in situ, the reducing agent Sodium triacetoxyborohydride (STAB) in the bioconjugation process of Pneumococcal conjugate vaccines (PCVs) [2]. In this application note, we demonstrate the use of Spinsolve 90 ULTRA spectrometer in the 1D 1H and 11B analysis with multinuclear decoupling techniques of different STAB mixtures in protonated DMSO solvent.
Experimental Setup
Sodium borohydride (NaBH4) when reacted with acetic acid (AcOH) results in the generation of H2 gas and the respective sodium acetoxyboro-hydride species depending on the acetic acid’s equivalents. (eq.) and reaction conditions as shown in Equation 1 [3].
Samples with different ratio of NaBH4 and acetic acid in protonated DMSO were prepared (Table 1). After the H2 gas generation had finished, the mixture was transferred to NMR tubes for analysis.
Table 1. Sample information
NMR Results & Discussion
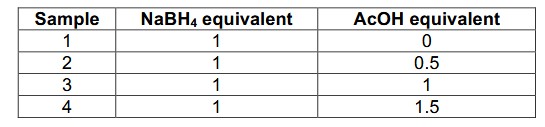
First, sample 1 was analyzed with 1D NMR techniques with and without decoupling for both 1H and 11B nuclei. The 1D 11B spectrum of NaBH4 (Fig. 1A) shows a quintet coupling pattern from the neighboring four protons. This 1H-11B coupling is removed in a 1D 11B{1H} experiment (Fig. 1B) to result in a singlet 11B signal with increased signal intensity, hence increased measurement sensitivity. Collapsing the signals in the 11B {1H} experiment can be essential when there are overlapping signals for analyzing newly formed boron species during the reactions.
In the 1H spectra (Figure 1B and 1C), a more complex splitting pattern is observed due to the coupling of 1H with both 10B and 11B NMR-active isotopes. Quadrupolar 11B (spin 3/2 with 80% isotopic natural abundance) couples to the neighboring protons in NaBH4 which results in a 1:1:1:1 quartet in the proton spectra between -2 and +1 ppm (11B satellites in Figure 1C). In this same spectrum, a less intense 1:1:1:1:1:1:1 heptet was also observed between -1.5 to +1 ppm due to 1H-10B coupling (spin 3 with 20% isotopic natural abundance). When 11B decoupling scheme is applied, the quartet collapses into a singlet, and leaves the heptet from 1H-10B coupling.
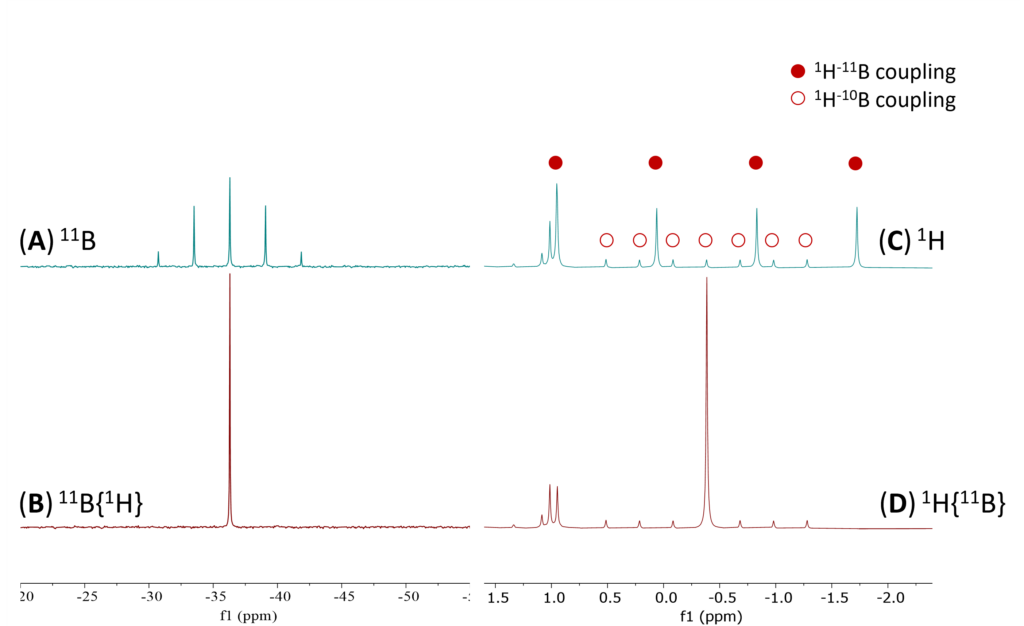
Figure 1. 1D spectra of sample 1 (NaBH4 in DMSO). (A) 1D 11B; (B) 1D 11B{1H}; (C) 1D 1H; (D) 1D 1H{11B}
The addition of acetic acid (0.5 equivalent in sample 2) generates a mixture of sodium acetoxyborohydride species (Figure 2) along with unreacted sodium borohydride. Adding to the spectral complexity, there is a clear overlap between one of the solvent DMSO’s 13C satellite peaks and the acetyl proton signals at 1.74 ppm. The tail of the solvent signal also distorts the baseline in this region. After applying 1D 1H{13C} WET solvent suppression (Figure 2 (blue)), the solvent signal intensity at 2.50 ppm was significantly reduced, which ensured the baseline resolution around the acetyl signals. Additionally, the 13C solvent satellite was removed. Therefore, the 1H{13C} with solvent suppression experiment was necessary and sufficient for a well-resolved and identifiable acetyl signals.
The 1D 1H{13C} with WET solvent suppression spectra of all AcOH/NaBH4 mixtures as well as the NaBH4 sample are compared in Figure 3. These spectra resemble the spectra of STAB mixtures in Merck’s publication [2]. This data shows that the relative ratio from the three singlets in the 1.75 ppm region, which corresponds to a mixture of acetyl groups, is changing when increasing AcOH. With just 0.5 equivalent of AcOH the relative ratio between the singlet was 1.0:2.5:1.1 respectively. When increasing to 1 and 1.5 equivalents, the ratios changed to 1.0:2.8:2.7 and 1.0:6.6:7.7 respectively. The groups at 1.74 and 1.78 have practically a 1:1 ratio with higher AcOH equivalents. In addition, the BH4 signals between -2 and +1 ppm disappeared completely in the sample with 1.5 equivalents AcOH. These spectral changes indicate that the NaBH4 was being consumed with increasing amount of AcOH while simultaneously the acetyl group was substituted onto the boron center.
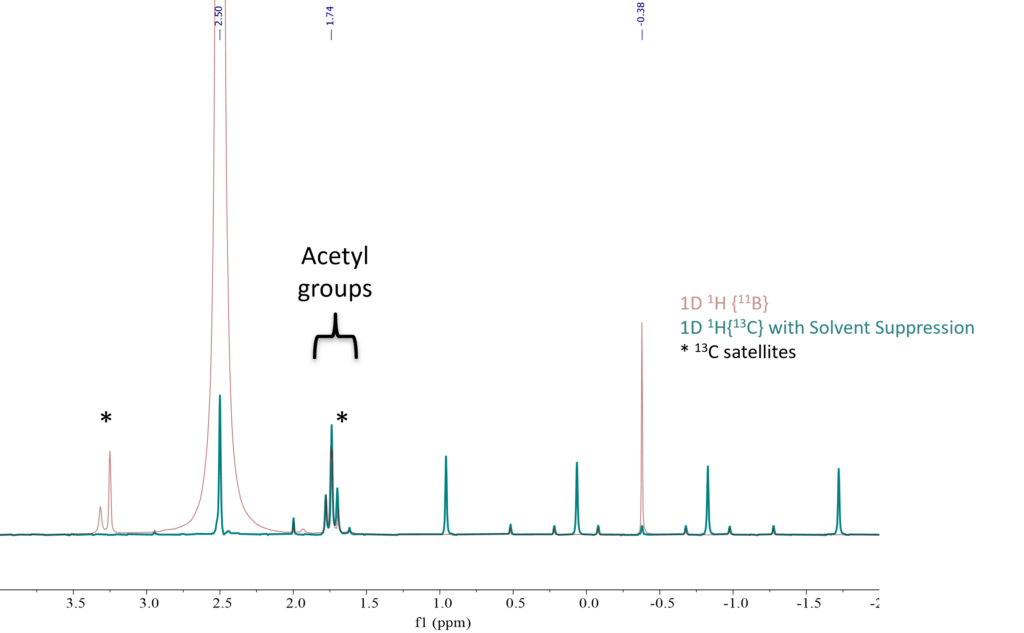
Figure 2. Superimposed spectra of sample 2 (0.5 eq. of AcOH) with and without WET solvent suppression (red) 1D 1H {11B} (blue) 1D 1H{13C} with Solvent Suppression
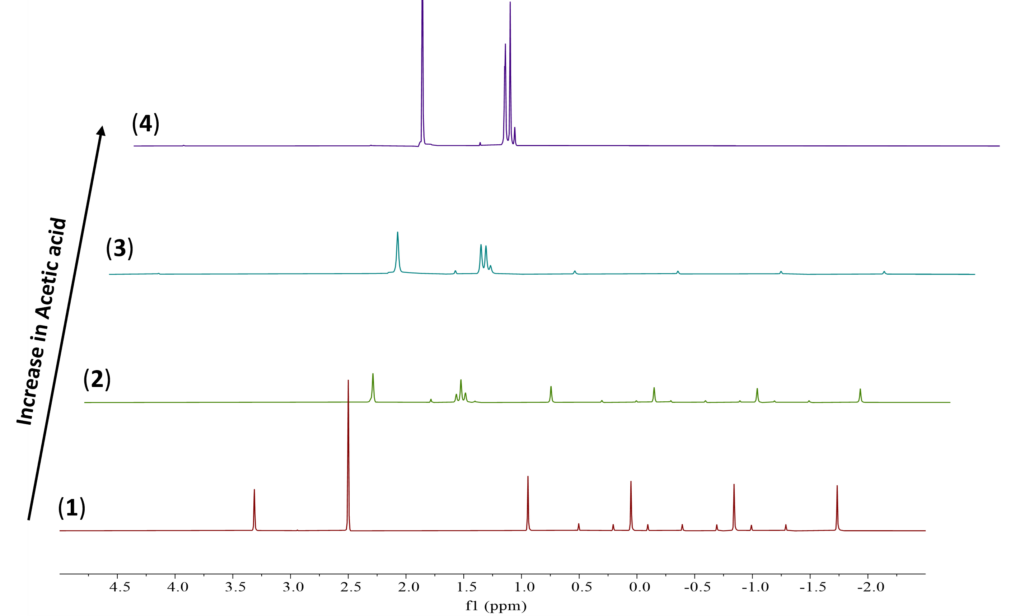
Figure 3. 1D 1H{13C} with solvent suppression stacked NMR spectra of (1) NaBH4 in DMSO, (2) 0.5 eq. of AcOH, (3) 1 eq. of AcOH, and (4) 1.5 eq. of AcOH
To ensure new boron species are formed, 1D 11B {1H} NMR experiments were conducted (Figure 4). The results corroborate the findings from the 1D 1H{13C} WET solvent suppression experiments. Two new boron signals, A and B, are observed in spectrum 2. When increased to 1.5 eq., a third boron signal, C, is observed in spectrum 4. The sharp boron signal from NaBH4 also disappeared entirely in spectrum 4. This disappearance indicates the consumption of NaBH4 in the reaction with AcOH in DMSO.
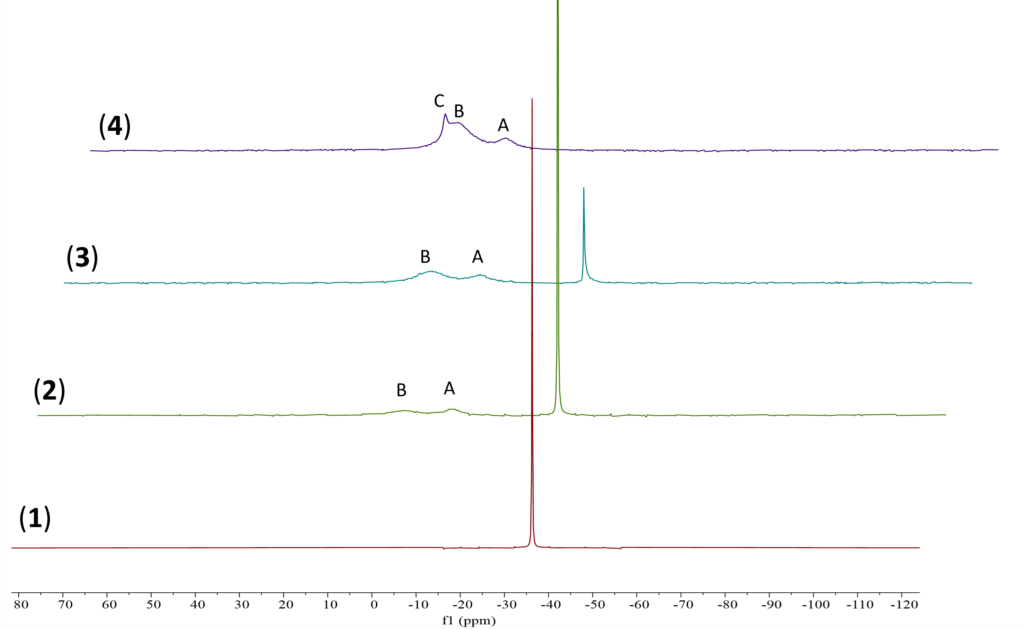
Figure 4. 11B{1H} stacked NMR spectra of (1) NaBH4 in DMSO, (2) 0.5 eq. of AcOH, (3) 1 eq. of AcOH, and (4) 1.5 eq. of AcOH
Conclusion
This application note demonstrates that the Spinsolve benchtop NMR offers a great technique for analyzing and monitoring boron species in reactions. Through the NMR experiments, 1D 11B {1H} and 1H{13C} WET solvent suppression, the signals of different sodium acetoxyboro- hydride species in the mixture were well-resolved, identified and can be quantified with practically no need for sample preparation. This enables a deeper understanding of reagents and providing additional attributes for chemical process control and optimization.
To read the complete App Note please Click Here
References
[1] M. TC and A. Jones, “Determination of Boron Content Using a Simple and Rapid Miniaturized Curcumin Assay,” Bio. Protoc., vol. 8, no. 2, 2018, doi: 10.21769/BioProtoc.2703.
[2] M. L. Smith et al., “Elucidating the Critical Attributes of Sodium Triacetoxyborohydride to Tune Glycoconjugation via Reductive Amination,” Bioconjug. Chem., vol. 36, no. 11, pp. 2381–2388, Nov. 2025, doi: 10.1021/acs.bioconjchem.5c00377.
[3] A. V. Panfilov, Yu. D. Markovich, A. A. Zhirov, I. P. Ivashev, A. T. Kirsanov, and V. B. Kondrat’ev, “Reactions of Sodium Borohydride in Acetic Acid: Reductive Amination of Carbonyl Compounds,” Pharm. Chem. J., vol. 34, no. 7, pp. 371–373, Jul. 2004, doi: 10.1023/A:1005221508362.