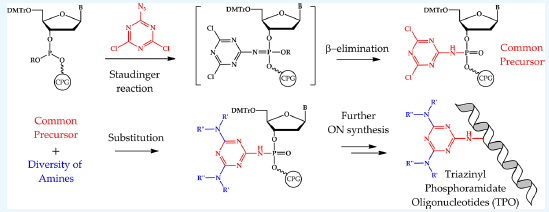
725. General Approach for the Synthesis of Triazinyl Phosphoramidate Oligonucleotides via the Staudinger Reaction with the Use of 2‑Azido-4,6-dichloro-1,3,5-triazine
Timofey D. Zharkov, Maxim S. Kupryushkin, ACSOmega, (2025), DOI: 10.1021/acsomega.5c06606
Developing the new types of phosphate-modified oligonucleotides and methods for their synthesis is of great interest in nucleic acid chemistry, since such derivatives could be applied as diagnostic and therapeutic tools. In the present study, our previously reported synthesis of oligonucleotide bearing two dodecyl residues on the triazine core was expanded to a general approach of synthesis of a novel class of phosphate-modified nucleic acids, triazinyl phosphoramidate oligonucleotides (TPO). The proposed approach for obtaining TPO is based on the Staudinger reaction between highly reactive 2-azido-4,6-dichloro-1,3,5-triazine and the phosphite triester intermediate of oligonucleotide solid-phase synthesis, with the following β-elimination of 2-cyanoethyl protective group and substitution of chlorine atoms with various amine residues. The formation of the triazinyl phosphoramidate bond was confirmed by carrying out 31P NMR of dithymidylate TPO and its comparison with other well-known PN-modified nucleic acid classes such as phosphoryl guanidine and alkyl and sulfonyl phosphoramidates. The possibility of great diversification of substituents in the triazine core of TPO using the proposed approach with incorporation of alkylamines, arylamines, amino alcohols, and diamines with a single substitution step as well as solid-phase multistep assembly of different complex residue structures was demonstrated. The obtained modified oligonucleotides were analyzed using high-performance liquid chromatography (HPLC) and mass analysis methods. Alternative structures of azido-triazine modifiers containing methylamino, methoxy groups, and iodine atoms were also proposed and studied for the efficiency of obtaining TPO. The presented results indicate that the described triazinyl phosphoramidate oligonucleotides are a distinct class of phosphate-modified nucleic acids with a broad range of representatives and a potentially adjustable approach for their synthesis.