108. Reaction Monitoring Using SABRE-Hyperpolarized Benchtop (1 T) NMR Spectroscopy
Olga Semenova, Peter M. Richardson, Andrew J. Parrott, Alison Nordon, Meghan E. Halse, and Simon B. Duckett, Analytical Chemistry, (2019) DOI: 10.1021/acs.analchem.9b00729
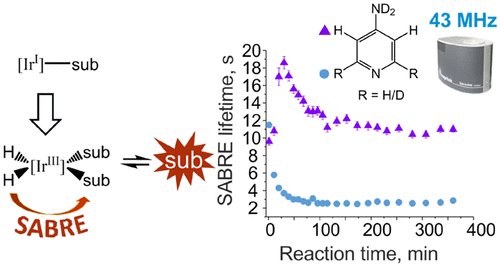
The conversion of [IrCl(COD)(IMes)] (COD = cis,cis-1,5-cyclooctadiene, IMes = 1,3-bis(2,4,6-trimethyl-phenyl)imidazole-2-ylidene) in the presence of an excess of para-hydrogen (p-H2) and a substrate (4-aminopyridine (4-AP) or 4-methylpyridine (4-MP)) into [Ir(H)2(IMes)(substrate)3]Cl is monitored by 1H NMR spectroscopy using a benchtop (1 T) spectrometer in conjunction with the p-H2-based hyperpolarization technique signal amplification by reversible exchange (SABRE). A series of single-shot 1H NMR measurements are used to monitor the chemical changes that take place in solution through the lifetime of the hyperpolarized response. Non-hyperpolarized high-field 1H NMR control measurements were also undertaken to confirm that the observed time-dependent changes relate directly to the underlying chemical evolution. The formation of [Ir(H)2(IMes)(substrate)3]Cl is further linked to the hydrogen isotope exchange (HIE) reaction, which leads to the incorporation of deuterium into the ortho positions of 4-AP, where the source of deuterium is the solvent, methanol-d4. Comparable reaction monitoring results are achieved at both high-field (9.4 T) and low-field (1 T). It is notable that the low sensitivity of the benchtop (1 T) NMR enables the use of protio solvents, which when used here allows the effects of catalyst formation and substrate deuteration to be separated. Collectively, these methods illustrate how low-cost low-field NMR measurements provide unique insight into a complex catalytic process through a combination of hyperpolarization and relaxation data.