140. On the aqueous chemistry of the U-IV-DOTA complex
Gev Dovrat, Marie-Claire Illy, Claude Berthon, Ana Lerner, Moshe Mintz, Eric Maimon, Radion Vainer, Yeshayahu Ben-Elyiahu, Yulia Moiseev, Philippe Moisy, Armand Bettelheim, and Israel Zilbermann, Chemistry – A European Journal, (2020) DOI: 10.1002/chem.201905357
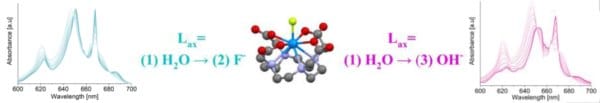
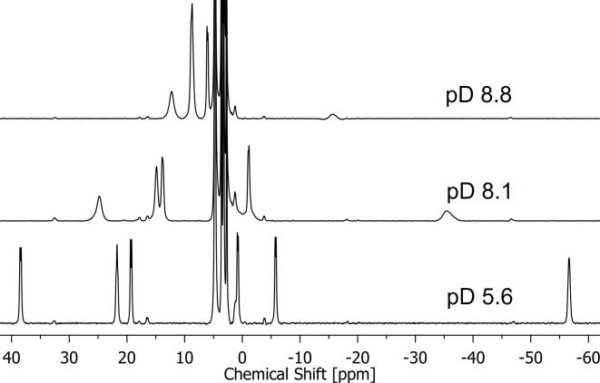
The 1,4,7,10-tetrazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) aqueous complex of UIV with H2O, OH- and F- as axial ligands was studied using UV-Vis-spectrophotometry, ESI-MS, NMR spectroscopy, X-ray Crystallography and electrochemistry. The UIVDOTA complex with either water or fluoride as axial ligands was found to be inert to oxidation by molecular oxygen while the complex with hydroxide as axially ligand slowly hydrolyzed and was oxidized by dioxygen to a di-uranate precipitate. The combined data set acquired shows that even though the axial substitution of fluoride and hydroxide ligands instead of water does not seem to significantly change the aqueous DOTA complex structure, it has an important effect on the complex electronic configuration. The UIV/UIII redox couple was found to be quasi-reversible for both axially H2O and hydroxide bonded complex but irreversible for the axially fluoride bonded complex. Intriguingly, axially fluoride binding renders the irreversible one-electron UV/UIV oxidation of the [UIVDOTA(H2O)] complex to be quasi-reversible, suggesting the formation of the short-lived pentavalent form of the complex- an aqueous non-uranyl chelated UV cation.