185. Solute Specific Perturbations to Water Structure and Dynamics in Tertiary Aqueous Solution
Harrison Laurent, Daniel L. Baker, Alan K. Soper, Michael E. Ries, and Lorna Dougan; The Journal of Physical Chemistry B; (2020); DOI: 10.1021/acs.jpcb.0c07780
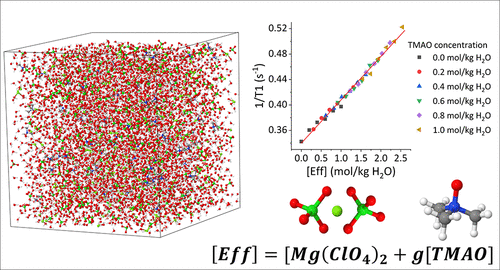
Liquid water is known as the “universal” solvent, capable of dissolving a wide variety of different solutes. While much is now understood about the impact of solutes on the water structure in binary solutions, it is much more challenging to deconvolute the potentially competing effects of more complex solutions. Here, we present a correlative NMR and neutron diffraction study to examine the solute induced perturbation of water structure and dynamics in a tertiary solution containing the naturally occurring osmolyte trimethylamine N-oxide (TMAO) and magnesium perchlorate (Mg(ClO4)2). We show that while TMAO and Mg(ClO4)2 perturb the water structure in an opposing manner, the two solutes slow water dynamics in an additive manner. We quantify the relative ability of each solute to perturb water by introducing a weighting parameter and show that TMAO is 1.54 times more effective at perturbing water structure and dynamics than Mg(ClO4)2. The combination of NMR, neutron diffraction, and computational modelling offers unprecedented access to the structure and dynamics of more complex aqueous solutions, permitting the deconvolution of solute specific perturbation of water. Such insight provides a new route to understand this universal solvent in the context of important and relevant aqueous environments.