216. Intensified Continuous Flow Synthesis and Workup of 1,5-Disubstituted Tetrazoles Enhanced by Real-Time Process Analytics
Peter Sagmeister, Dainis Kaldre, Joerg Sedelmeier, Christian Moessner, Kurt Püntener, Dominique Kummli, Jason D. Williams, and C. Oliver Kappe; Organic Process Research & Development; (2021); DOI: 10.1021/acs.oprd.1c00096
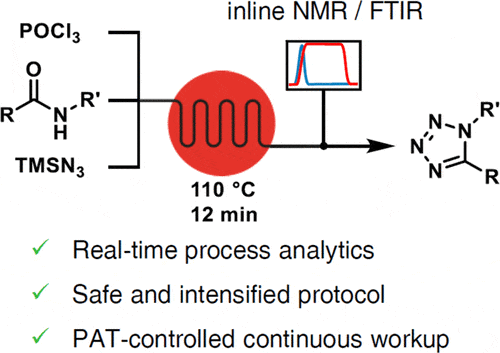
Continuous flow processing presents a solution for the safe and effective formation of 1,5-tetrazoles due to the small reactive inventory and absence of a reactor headspace. This has been exemplified using a model amide, 2-chloro-N-methylacetamide, activated using POCl3 to its corresponding imidoyl chloride, which reacts with trimethylsilyl azide. Initial scoping revealed that an excess of azide is vital to facilitate a clean reaction but also that a high concentration would dramatically accelerate the reaction without the need for greatly elevated temperature. In a short residence time of 10 min, complete conversion was achieved, resulting in 77–86% yield of the desired product in high purity (>99.7%) after recrystallization, omitting any chromatographic purification. The developed process reached an excellent space–time yield (1.16 kg L–1 h–1) due to its high concentration and short residence time. Successful technology transfer included development of a continuous workup, with pH-controlled quench in a CSTR, followed by extraction. During flow optimization, two orthogonal PAT methods were separately employed, NMR and FTIR, enabling real-time starting material and product quantification. Finally, the developed reaction protocol was demonstrated on a number of other substrates, achieving high yields in most cases, up to quantitative.