275. Intensified Continuous Flow Michaelis–Arbuzov Rearrangement toward Alkyl Phosphonates
Thomas Toupy and Jean-Christophe M. Monbaliu; Organic Process Research & Development; (2022); DOI: 10.1021/acs.oprd.1c00472
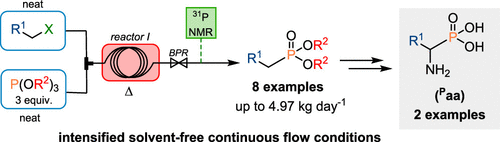
Herein is described the development of an intensified continuous flow process for the preparation of a library of alkyl phosphonates through a Michaelis–Arbuzov rearrangement. A careful process optimization and thorough analysis of the competitive reactions led to a very attractive protocol with unprecedented productivities (up to 4.97 kg of material per day) and a low environmental footprint with the absence of solvent, additives, catalysts, and waste. In-line low-field 31P NMR monitoring was conveniently implemented for rapid optimization and process monitoring. Two key alkyl phosphonate intermediates were also assessed for the unprecedented diazene dicarboxylate-mediated electrophilic amination under continuous flow conditions toward the α-aminophosphonic acid derivatives of Pphenylalanine and Palanine, bioisosters of the natural amino acids phenylalanine and alanine, respectively.