373. Selective Enzymatic Esterification of Lignin-Derived Phenolics for the Synthesis of Lipophilic Antioxidants
Marta Martinez-Garcia, Jaime Gracia-Vitoria, Karolien Vanbroekhoven, Winnie Dejonghe and Yamini Satyawali; Antioxidants (2023); DOI: 10.3390/antiox12030657 (open access)
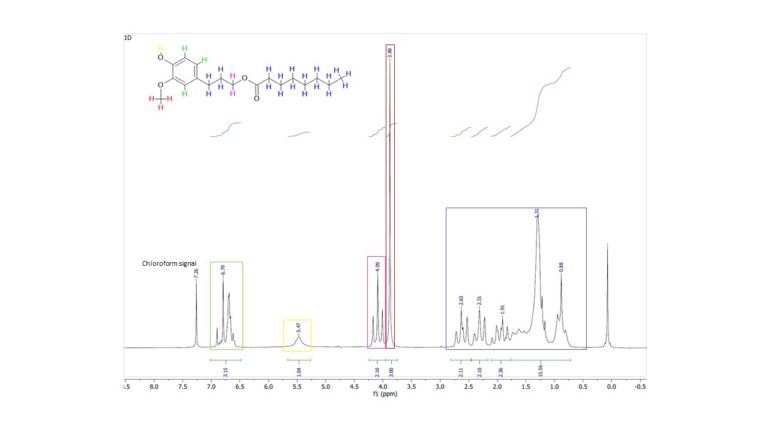
Lignin is an abundant and renewable source of phenolic compounds that can be used as natural antioxidants to substitute synthetic, petroleum-based alternatives. The development of lignin depolymerization techniques has improved the accessibility of low-molecular-weight phenolic fractions with enhanced antioxidant activity compared to native lignin. The selective esterification of the aliphatic OH groups in these compounds is necessary in order to increase their compatibility with hydrophobic product matrixes, while preserving their antioxidant capacity. In the present work, lipase was chosen as a selective catalyst for the esterification of the monolignol dihydroconiferyl alcohol (DCA), in order to target the esterification of aliphatic OHs without modifying the aromatic groups. The reaction was studied under solvent-assisted and solvent-free conditions, using different fatty acids and substrate ratios. A product yield of 97% could be obtained after 24 h in a solvent-assisted reaction with 2 molar equivalents of fatty acid, or after 3 h in a solvent-free reaction with 10 molar equivalents of the fatty acid. The esterified monolignol showed relevant long-term radical scavenging activity, comparable to other commercial, petroleum-based antioxidants. Different lignin fractions were also used as substrates for enzymatic esterification with different fatty acids, resulting in esterification degrees of 20–58% (of the total aliphatic OH), depending on the specific combination of fatty acid–lignin fractions.