626. H₂ Production from Photocatalytic Reforming of PET over Pt/TiO₂: The Role of Terephthalic Acid
Luke Roebuck, Min Hu, Helen Daly, Hubertus Warsahartana, Louise S. Natrajan, Arthur Garforth, Carmine D’Agostino, Marta Falkowska, Christopher Hardacre, CatalysisToday, (2025), DOI: 10.1016/j.cattod.2025.115242
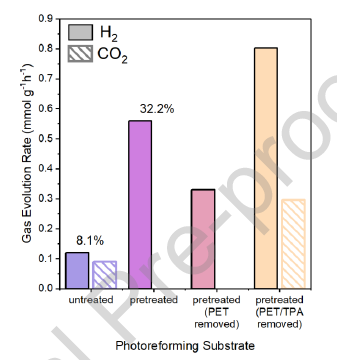
Photoreforming is a promising method for the conversion of waste materials with simultaneous production of H2. The use of waste polyethylene terephthalate (PET) as a photoreforming substrate has been previously investigated, however its insolubility in aqueous media and the resistance of the aromatic terephthalate towards conversion are major obstacles. Commonly an alkaline pretreatment step is used to initiate hydrolysis to ethylene glycol and terephthalic acid which promotes H2 evolution. However, in this work we have found that TPA has both promotional and inhibitory effects by modification of the catalyst surface that depend on the relative concentration of ethylene glycol. Terephthalic acid inhibits the oxidation reactions by scavenging hydroxyl radicals and blocking complexation sites. This leads to lower H2 evolution compared to the photoreforming of an equivalent concentration of ethylene glycol. Even in trace amounts, terephthalic acid would still inhibit the reaction unless the concentration of ethylene glycol was high enough. Surprisingly, at ethylene glycol concentrations of >1.2 M, residual terephthalic acid promoted the reaction which is thought to be due to increasing the interaction between ethylene glycol and the catalyst surface but also an increased role of water. On the basis of these results, we suggest that, if PET is to be used as a feedstock for H2 generation by photoreforming, an initial hydrolysis should be performed after which terephthalic acid is separated for re-use. The remaining hydrolysate may then be used for photoreforming. Furthermore, the ethylene glycol concentration should be maximized in order to overcome the inhibitory effects of residual terephthalic acid.