637. Synthesis of ¹⁴C-Labeled Polyethylene Terephthalate and Generation of ¹⁴C-Nanoparticles for Fate and Disposition Studies
Anuradha Singh, Weilin L. Shelver, David J. Smith, JLabeledCompRadiophar, (2025), DOI: 10.1002/jlcr.4137
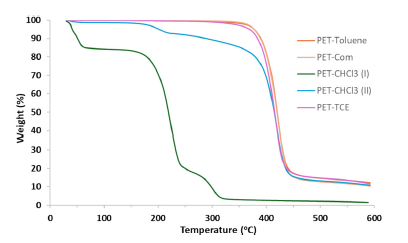
Polyethylene terephthalate (PET) is one of the most extensively used plastics in daily life. Due to its prevalent use, it is ubiquitous in the environment and a significant contributor to plastic pollution. Continuous exposure to photochemical, thermal, biological, and mechanical processes makes PET susceptible to slow degradation and the production of microsized and/or nanosized particles known as PET microplastic/nanoplastic (MP/NP). MP/NP are widely detected in the environment, including in drinking water and human food; consequently, knowledge gaps on the impacts of MP/NP in human food sources have gained global attention. A large knowledge gap is the bioaccumulation and fate of PET MP/NP in food animals. The application of carbon-14 labeled PET NP in food animals would provide a relatively straightforward approach to understanding the degree of PET absorption and its tissue distribution after absorption. Here, a simple, fast, and efficient synthetic method is described to produce [14C]-PET NP. The method comprises the polycondensation of terephthaloyl chloride and readily accessible [14C]-ethylene glycol followed by nanoprecipitation. The synthesized [14C]-PET and [14C]-PET NP were characterized by nuclear magnetic resonance spectroscopy (NMR), Fourier transform infrared spectroscopy (FTIR), dynamic light scattering spectroscopy, thermogravimetric analyzer (TGA), and UV-Vis spectroscopy.