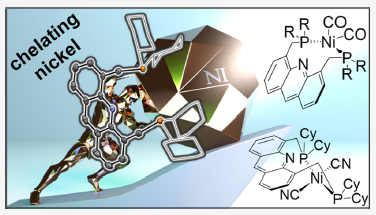
647. Nickel Complexes of 4,5-Bis(diorganophosphinomethyl)acridine Ligands
Maximilian Roca Jungfer, Frank Rominger, Thomas Oeser, A. Stephen K. Hashmi, Thomas Schaub, Organometallics, (2025), DOI: 10.1021/acs.organomet.5c00033
In a study to expand the coordination chemistry of methylene-extended 4,5-bis(diorganophosphinomethyl)acridine ligands (LR) to first-row transition metal ions, bidentate P,P-chelated nickel complexes of these usually tridentate pincer ligands were identified. Similar to the iron complexes of these ligands, the acridines’ nitrogen atom does not coordinate to nickel, leading to the formation of nickel bisphosphine-type complexes adopting both cis- and trans-coordination modes. Nickel complexes with compositions [NiX2(κ2-LR)] (X = Cl, Br, I, CO, CN, and R =Cy, iPr) were isolated and characterized. The bisphosphine ligands are weakly bound to nickel and can be transferred from [NiCl2(κ2-LR)] to [ClAu(SMe2)], giving the gold(I) cations [Au(LR)]+. The chlorido ligands are selectively exchanged for CN− during the reaction of [NiCl2(κ2-LR)] with KCN, giving trans-[Ni(CN)2(κ2-LCy)]. In contrast, the nickel(0) carbonyls [Ni(CO)2(κ2-LR)] form in photochemical reactions from [Ni(CO)2(PPh3)2] and LR in partially reversible reactions and are intrinsically unstable in solution.