659. Stabilities and Limitations in the Reactivity of Phosphorus Ylide-Based Aluminum- and Gallium-Carbon Ambiphiles − A Combined Experimental and Computational Approach
Felix Krämer, Pascal Weisenburger, Israel Fernández, Frank Breher, EurJlnoryChem, (2025), DOI: 10.1002/ejic.202500165
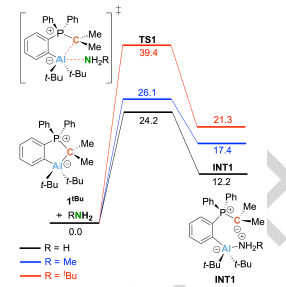
The unexpected reactivity and stability limits of phosphorus ylide-based aluminum- and gallium-carbon ambiphiles are described. While the previously published t-butyl-substituted compound (2-{AltBu2}-C6H4)Ph2PCMe2 (1tBu) reacts reversibly with NH3 at room temperature with cleavage of a N–H bond, the reaction with MeNH2 is much less favourable and proceeds irreversibly only at 90 °C. All other title compounds 1R with R = Me, Et, Mes, and C6F5 decomposed in the presence of NH3. The decomposition of 1Et in the presence of ammonia can be well followed by NMR spectroscopy. All title compounds remain stable in the presence of t-BuNH2 and Et2NH. In addition, we found an unexpected reactivity in the reaction of 1R and the gallium analogues 2R with isocyanates. Instead of yielding the expected ring-expansion products, the title compounds catalyse the trimerization of isocyanates. We also present the reactivity towards MeOH and H2O. Quantum chemical calculations show that activation of the O–H bonds should be feasible at room temperature. Experimental findings, however, only show the decomposition of 1tBu in the corresponding reactions. Nevertheless, the cleavage of the O–H bond is feasible and affords the activation products 7 and 8 starting from the ammonia activation product 3.