729. Elucidating the Critical Attributes of Sodium Triacetoxyborohydride to Tune Glycoconjugation via Reductive Amination
Mackenzie L. Smith, Sarah Sirajuddin, Adriana N. Santiago-Miranda, Richard R. Rustandi, Jacob H. Waldman, Mikhail Reibarkh, Joseph P. Smith, Patrick M. McHugh, BioconjugateChemistry, (2025), DOI: 10.1021/acs.bioconjchem.5c00377
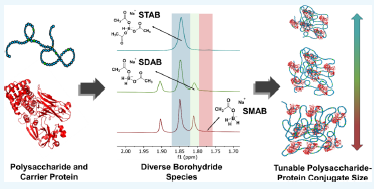
Pneumococcal conjugate vaccines (PCVs) have effectively enhanced immunogenicity by conjugating a carrier protein to a purified capsular polysaccharide. The degree of conjugation influences the effective size of the final conjugate, and control of this reaction is critical in developing a robust process. Sodium triacetoxyborohydride (STAB) is a common reducing agent used to perform reductive aminations to provide a means for conjugation and can be utilized as an in situ preparation in the PCV conjugation process. Robust analytical methods for characterizing STAB were not previously available. Herein, we develop methods to rapidly assess STAB for both activity and composition using quantitative NMR methodologies and apply these learnings to improve our understanding of the bioconjugation process. It was determined that decreasing the reaction temperature to synthesize STAB resulted in a more active reducing reagent enriched with sodium diacetoxyborohydride (SDAB). Conjugation reactions performed with a model polysaccharide and carrier protein found that an increased SDAB content led to larger conjugation sizes. Moreover, we established a correlation between the conjugate size and SDAB concentration by charging the reaction with varying molar equivalents of SDAB. Through this work, a deeper understanding of the critical attributes of STAB was developed using diverse analytical methods, and these learnings can be applied to develop a more appropriate control strategy for producing glycoconjugate therapeutics.