94. Differentiation of fentanyl analogues by low-field NMR spectroscopy Jonathan Duffy, Aaron Urbas, Matthias Niemitz, Katrice Lippa, Ioan Marginean, Analytica Chimica Acta, (2019) DOI: 10.1016/j.aca.2018.12.014
Jonathan Duffy, Aaron Urbas, Matthias Niemitz, Katrice Lippa, Ioan Marginean, Analytica Chimica Acta, (2019) DOI: 10.1016/j.aca.2018.12.014
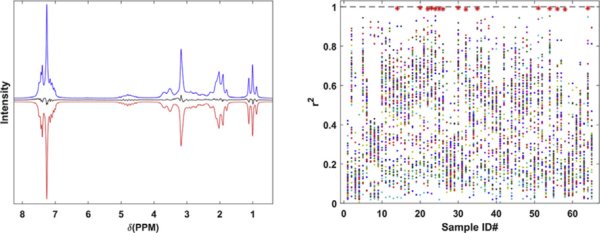
Forensic laboratories commonly receive new psychoactive substances such as fentanyl analogues and other synthetic opioids that are difficult to identify. Slight changes to chemical structures, e.g. shifting the position of functional groups such as methyl groups or halogens on the aromatic ring, may not be distinguished using traditional methods. NMR is a powerful tool used to elucidate distinctive structural information needed to differentiate regioisomers. However, the cost, size, and cryogen maintenance of superconducting NMR spectrometers can be impractical for some forensic laboratories. Recent studies have shown potential applications of low-field NMR as an alternative in forensic drug analysis. These benchtop, semi-portable instruments are less costly, have a smaller footprint, do not use cryogens, and require little maintenance. In this study, we show that 65 fentanyl and related substances, including various types of positional isomers, were readily differentiated using low-field (62 MHz) 1H NMR spectroscopy. In addition, the use of quantum mechanical spin system analysis was investigated for the purposes of translating experimentally observed high-field 1H spectra to lower field strengths. Spin system analysis of 600 MHz NMR spectra was conducted on a subset (15) of the reference materials analyzed. The results were used to calculate 62 MHz spectra for comparison purposes with the experimental spectra. This was successfully demonstrated, showing that field-strength independent 1H NMR spectral libraries are feasible and can facilitate reference material data dissemination across forensic drug laboratories.