With the growing market for electric and hybrid cars, a major focus of scientists today lies on the development of more efficient rechargeable batteries. In these devices, lithium salts are used as electrolytes dissolved in room-temperature ionic liquids (RTILs). A good understanding of the transport properties in the electrolytes is essential for the optimization of the performance of the batteries.
The Spinsolve systems can be equipped with a pulsed field gradients (PFG) to investigate such transport processes via their diffusional behaviour. As Spinsolve systems can be used to detect three different nuclei without the need of any hardware adjustment self-diffusion coefficients can be measured in a simple way of the different species present in the electrolyte mixture.
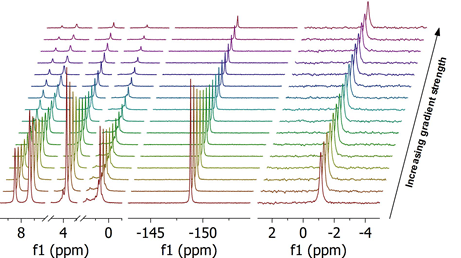
In the application described here we demonstrate the possibilities to measure self-diffusion coefficients of ions in mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate and lithium tetrafluoroborate via 1H, 19F and 7Li. One of the PFG experiments is shown in following figure (from left to right: proton, fluorine, and lithium):
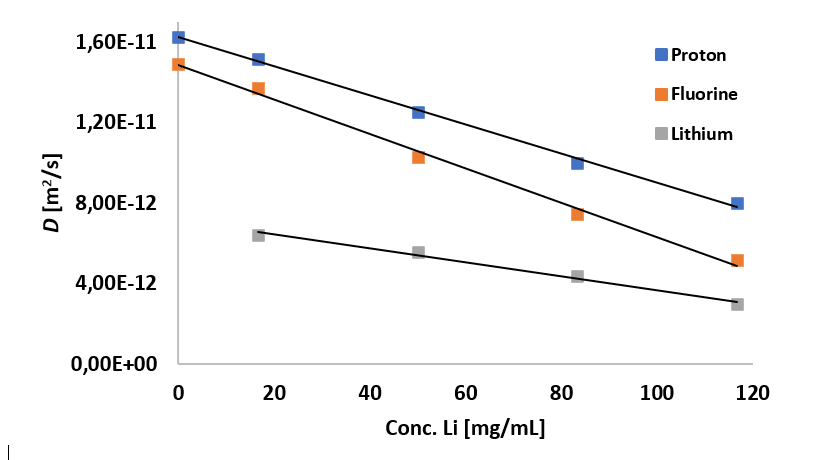
By analysing five different samples, a linear dependence between the lithium concentration and the self-diffusion coefficients was observed.
Read the full application note here: