Deuterium (2H or D), an isotope of hydrogen can be readily detected by NMR spectroscopy. Deuterium NMR has a chemical shift range similar to proton NMR but with a reduced resolution or in other words, deuterium signals have a broader natural line shape compared to their proton counterparts.
2H NMR can be used to verify the effectiveness of the process of deuteration, as a deuterated compound will be identified by peaks in deuterium NMR as well as the disappearance of signals in proton NMR. Deuterium NMR is also used as a potential dummy system for tritium 3H as tritium is not commonly measured by NMR due to its radioactivity.
Figure 1 shows the 2H spectra of the most common deuterated solvents used for NMR spectroscopy. All spectra were collected in a single scan.
Figure 1: 1D deuterium measurements of different deuterated solvents each measured with 1 scan, 5 seconds total measurement time
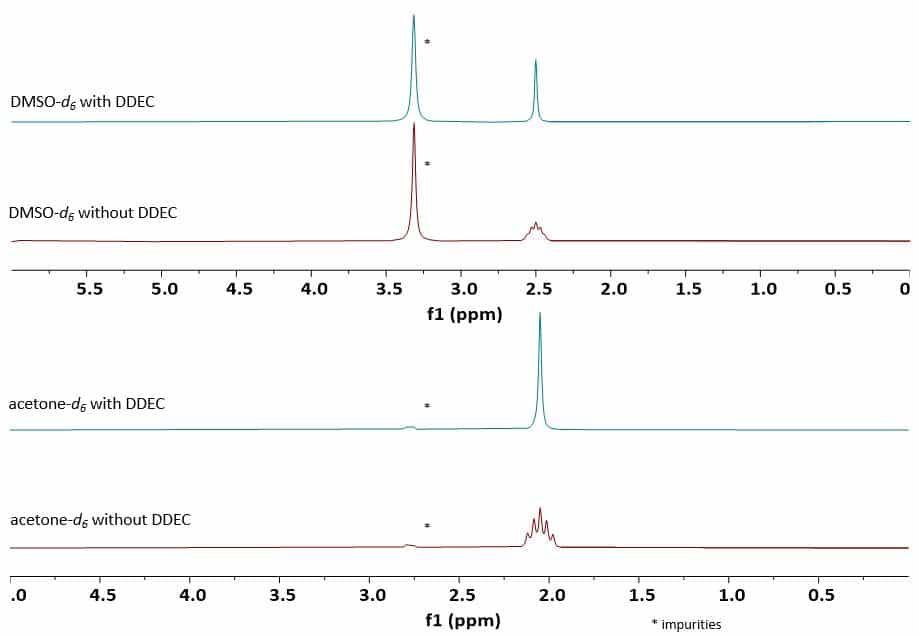
Figure 2 shows the proton spectra of DMSO-d6 and acetone-d6 measured with and without deuterium decoupling. Both spectra show the typical quintet that most NMR users have become familiar with. The quintet structure is due to the coupling of a residual proton (DMSO-d5, acetone-d5 ) coupled to the two adjacent deuterium on the same methyl group. Hence Deuterium has Spin I = 1, the multiplicity can be calculated as 2I x N + 1 = 2 x 1 x 2 + 1 = 5. Obviously, by applying deuterium decoupling the quintet collapses into a singlet, which can be useful, when you want to integrate a signal, which might otherwise overlap with the broader multiplet.
Figure 2: 1D 1H spectra of DMSO-d6 measured with and without deuterium decoupling (top); 1D 1H spectra of acetone-d6 measured with and without deuterium decoupling (bottom)