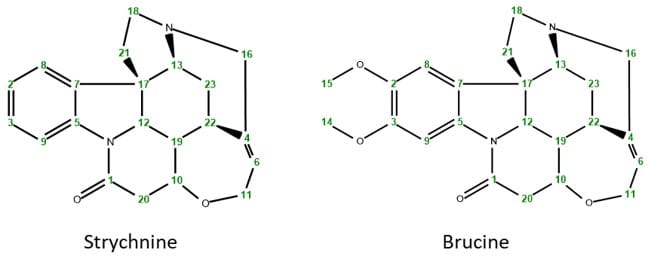
In a previous case study we investigated the structure of Brucine using different homo- and heteronuclear NMR techniques. Brucine in fact is an alkaloid closely related to the much better known strychnine, which is used as a pesticide, but is as well highly toxic for humans and other vertebrates. The two molecules only differ in the two methoxy groups at the aromatic ring that are present in Brucine but not in Strychnine.
Scheme 1: Chemical structures of strychnine and brucine
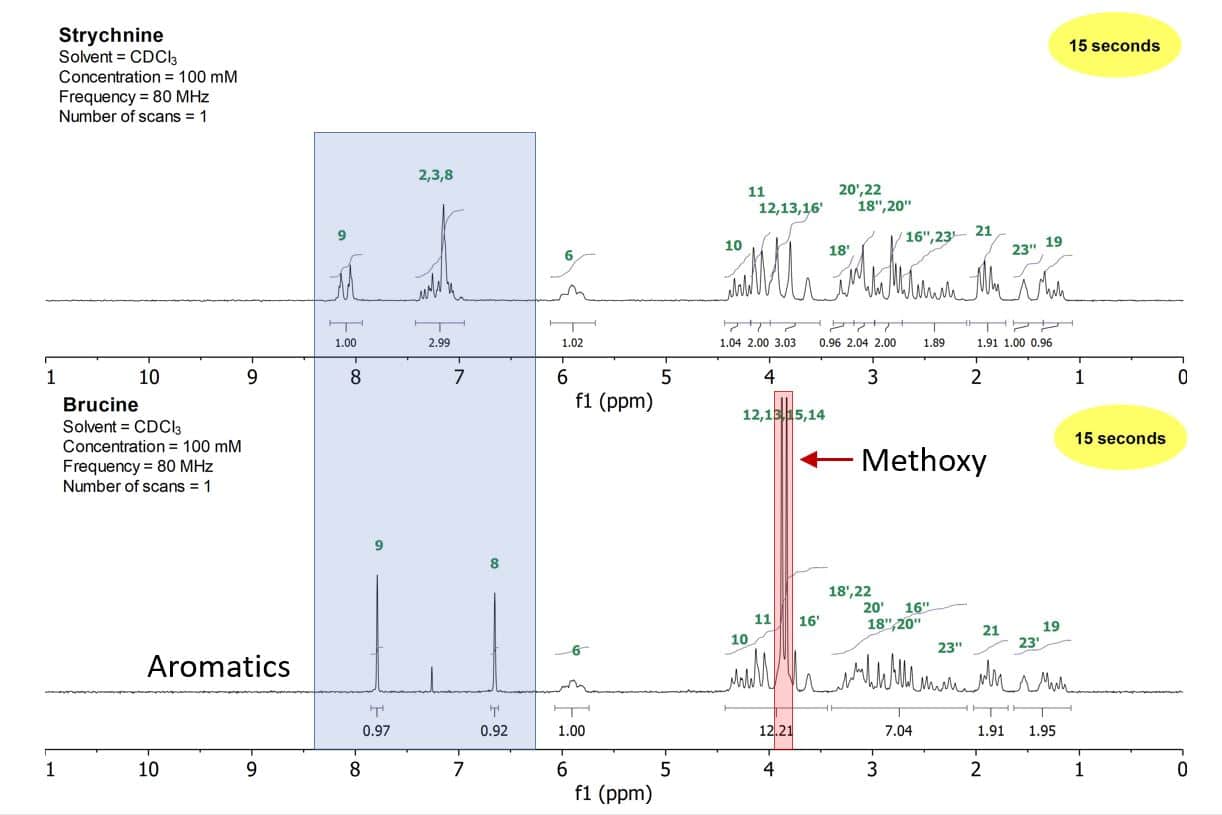
However, even these small differences lead to simply detectable changes in the NMR spectra of these two compounds. Figure 1 shows the 1D proton spectra of strychnine (top) and brucine (bottom). While the aliphatic region between 0–3.5 ppm stays quite similar, despite the two large methoxy signals at around 3.75 ppm, the aromatic region changes and simplifies substantially when going from strychnine to brucine.
Figure 1: 1D proton stack plot of a 100 mM strychnine sample (top) and a 100 mM brucine sample (bottom) both in CDCl3
With their superb resolution, the Spinsolve benchtop spectrometers enable not only a structural verification of organic compounds but as well allow for fingerprinting certain unknown substances, e.g. in forensic labs. This example of identifying two toxic compounds nicely underlines this application as it is possible to discriminate even based only on certain parts of the spectra.
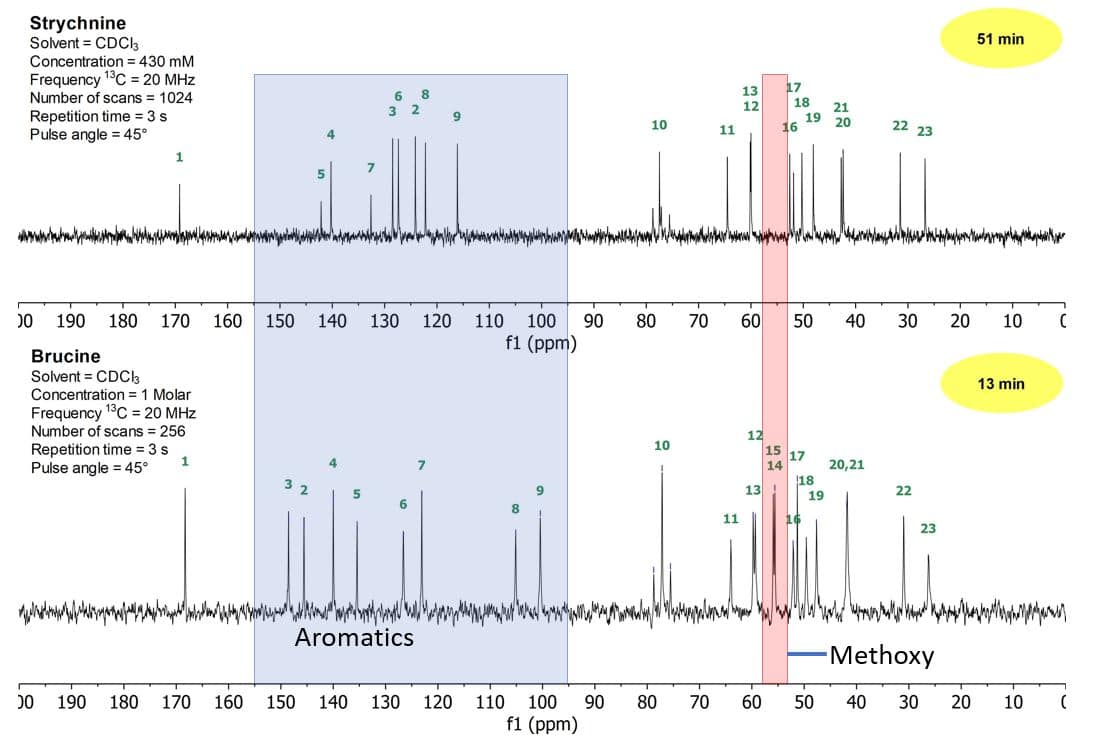
Figure 2 shows the 1D carbon spectra of strychnine (top) and brucine (bottom). As observed in the proton spectra, the aliphatic region of the carbon spectra between 20–80 ppm stays similar, while the aromatic region between 100–150 ppm changes substantially. The largest differences can be seen in the region between 100 and 150 ppm, where almost all signals change position.
Figure 2: 1D carbon stack plot of a 430 mM strychnine sample (top) and a 1 M brucine sample (bottom) both in CDCl3
Find a detailed comparison including 2D homo- and heteronuclear experiments run on these two compounds, download the following case study.