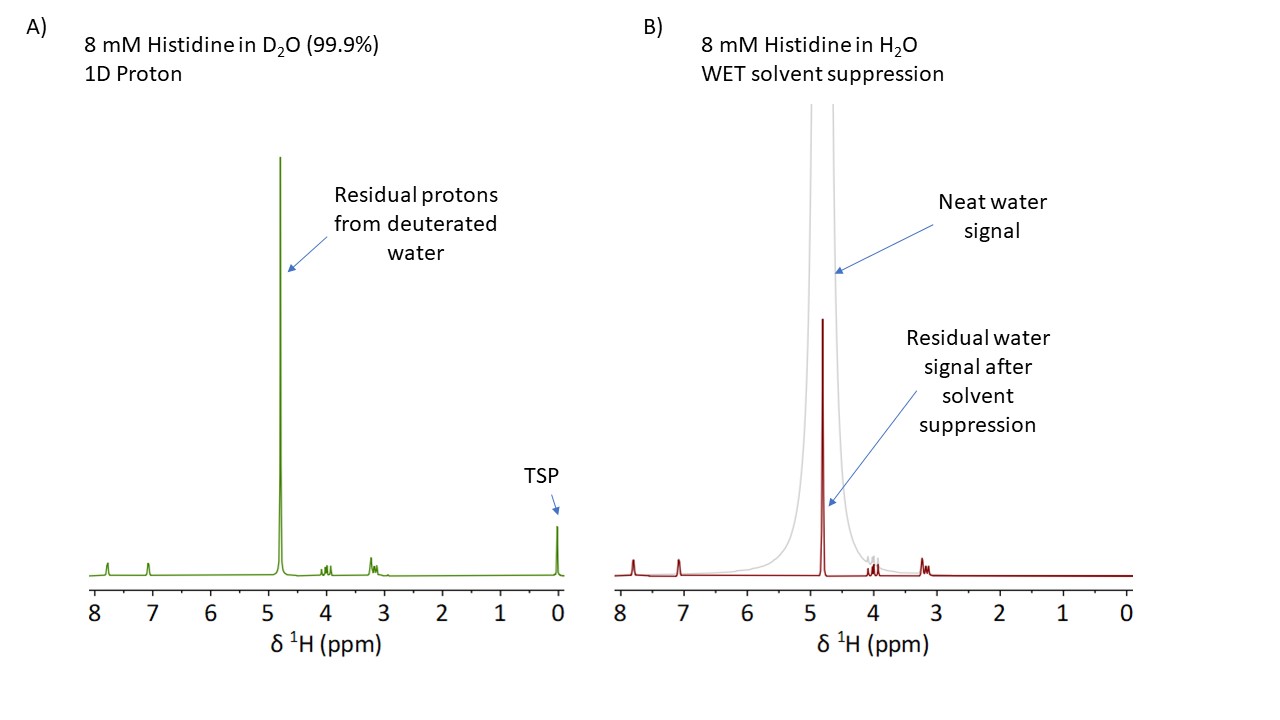
Typically, NMR samples are dissolved in deuterated solvents to avoid the overlapping of the large solvent peaks with the signal of the analytes. However, if samples need to be analyzed while they are being synthesized or if the components of a liquid formulation need to be quantified the use of deuterated solvents is not an option. In these situations, the alternative known from high field NMR is to implement solvent suppression methods that are used to drastically attenuate the solvent signals. On benchtop NMR systems, however, it is challenging to obtain efficient suppression as these methods require a highly homogeneous magnetic field, like the ones generated by superconducting magnets. This is what we have achieved with our Spinsolve ULTRA models, as we explain in our previous blogpost.
Here, we demonstrate how high the level of suppression can be on a Spinsolve ULTRA by comparing the residual signal of the solvent suppression with the signal of the residual protons present in deuterated solvents typically used for NMR. Figure A) shows a spectrum of 8 mmol histidine dissolved in 99.9 % deuterated water. The small concentration of the sample was chosen on purpose to better appreciate the residual signal of the solvent. Figure B) shows the comparison of the spectra measured with and without solvent suppression on an 8 mmol sample dissolved in regular H2O. By comparing the amplitude of the water signal we can determine that the suppression factor is about 1000.
As the scale on both figures is the same, you can see that the size of the residual signal of the protonated solvent is comparable to the signal of the residual protons in the deuterated solvent. This shows that the high quality of the solvent suppression makes it possible to measure samples in protonated solvents like if they were dissolved in deuterated solvents. In this way, you can measure your reaction mixture without the need for sample work up and solvent exchange. The one difference to keep in mind is that if the analyte has signals at the position of the solvent, then both signals will be suppressed. However, as it can be seen in Figure B), the suppressed region is typically narrower than 0.2 ppm.