Home Products Spinsolve family Spinsolve addons Sample Temperature Control
Download Resources
The Spinsolve systems can be equipped with a unique temperature control system that allows to control the temperature with out the need of additional nitrogen or dry air supply. This is achieved by adjusting the probe temperature instead of using a gas flow approach, which reduces the sample volume and sensitivity. The variable temperature option does not compromise resolution, sensitivity or stability.
Features:
- System and magnet design that allows to adjust the temperature of the probe
- Precise temperature control for static samples as well as online setups
- Works with standard 5 mm tubes and flow cell without sensitivity loss
- No requirement to re-calibrate at different temperatures
- No need of nitrogen or dry air supply
- Temperature range : RT to 60 °C
- Available as Standard and Ultra version
- Available with diffusion gradient
Excellent system stability
By utilizing the multi-layer temperature control of the Spinsolve series the system stability is not compromised over the whole temperature range.
Graph: 100 superimposed vegetable oil spectra taken over a period of 20 h at 50°C. Spectra were acquired without any reshimming between measurements
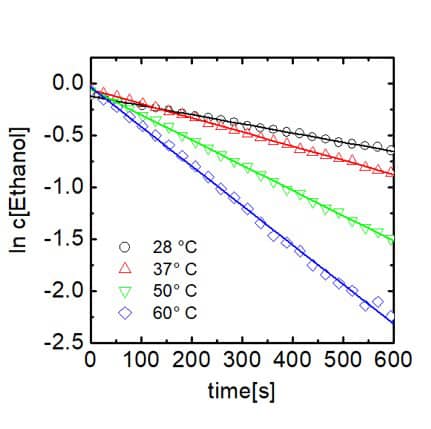
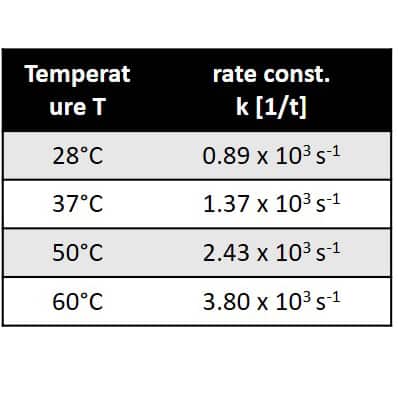
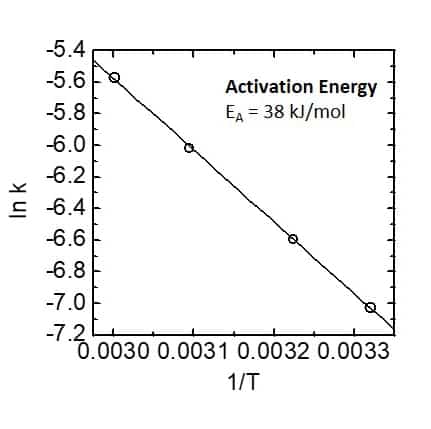
As trifluoro acetic acid was used in high excess the reaction follows a pseudo first order kinetics, which is demonstrated by linear dependence of the logarithm of the ethanol concentration on time. From the slopes of the linear fit the reaction rate constants can be extracted. Rate constant are given in the table in the center. Furthermore, when plotting the logarithm of the rate constant against T -1 and applying the Arrhenius equation the activation energy of the reaction can be determined via the slope of the linear dependence. This is demonstrated in the graph on the right hand side. 1
Improving resolution
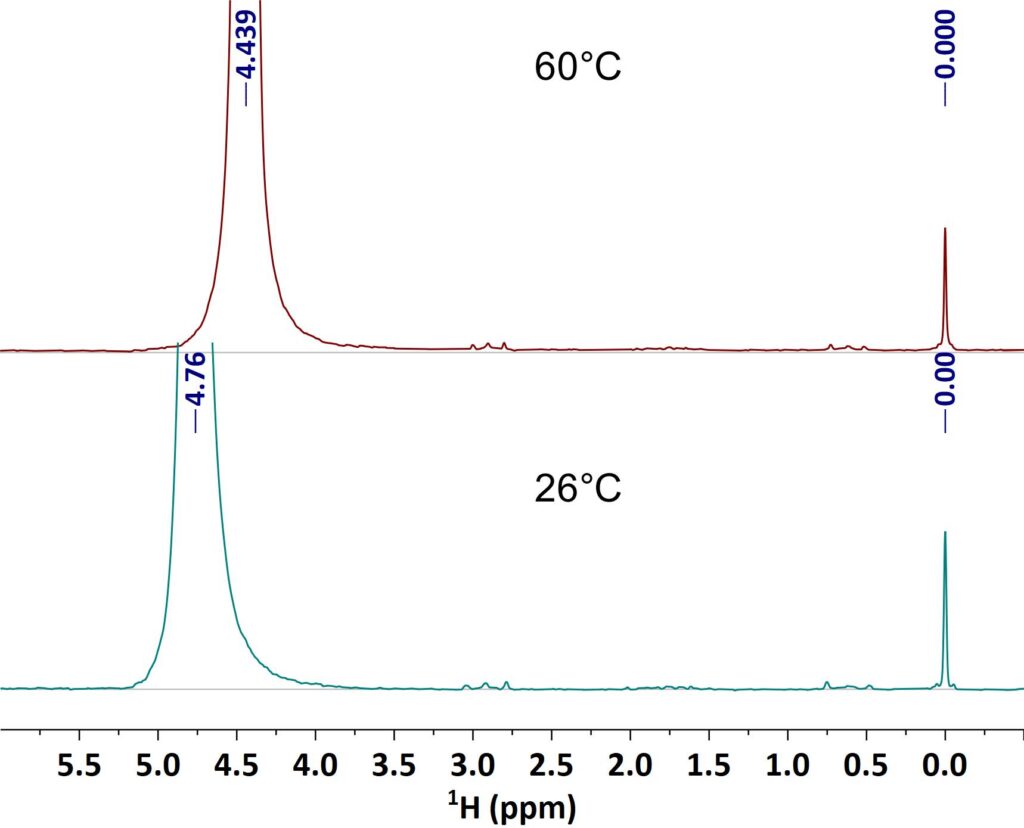
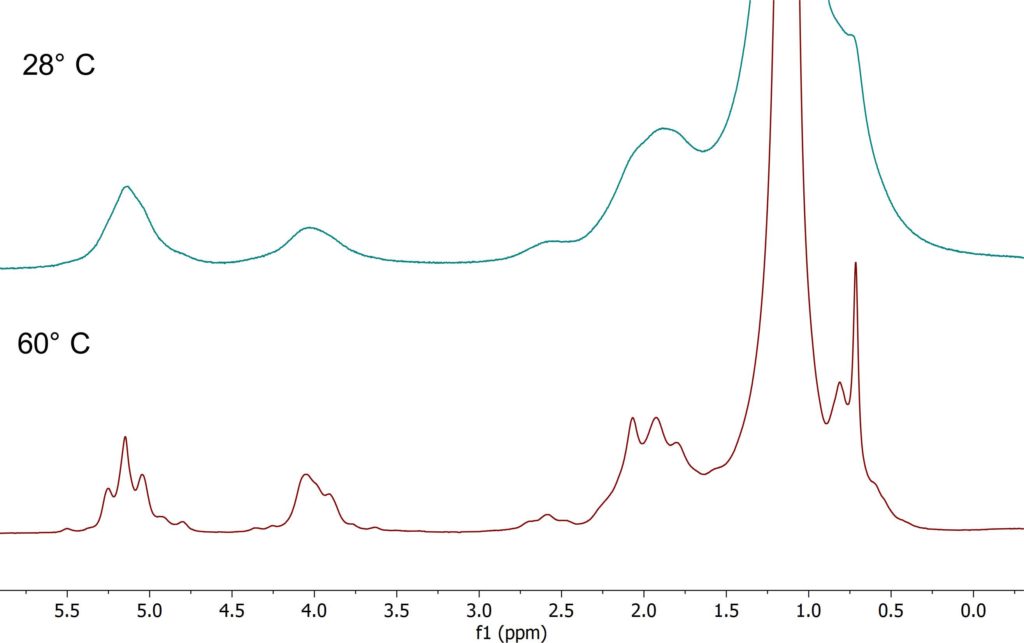
The linewidth in the NMR spectrum does not only depend on the homogeneity of the magnetic field, but as well on sample properties like the viscosity as well. High viscous samples do have shorter relaxation times resulting in broad lines. With increasing sample temperature the viscosity gets smaller and the lines in NMR spectrum get narrower. The example shows spectra of a vegetable oil taken at 28° C and 60° C respectively. It can be clearly seen how the linewidth improves at 60°C and the J-coupling patterns become visible.
The systems with the sample temperature control feature can as well be equipped with a pulsed field gradients. This enables one for example to run temperate dependent self-diffusion studies.