App Note – 1H-15N HSQC of GB1 protein measured at 80 MHz
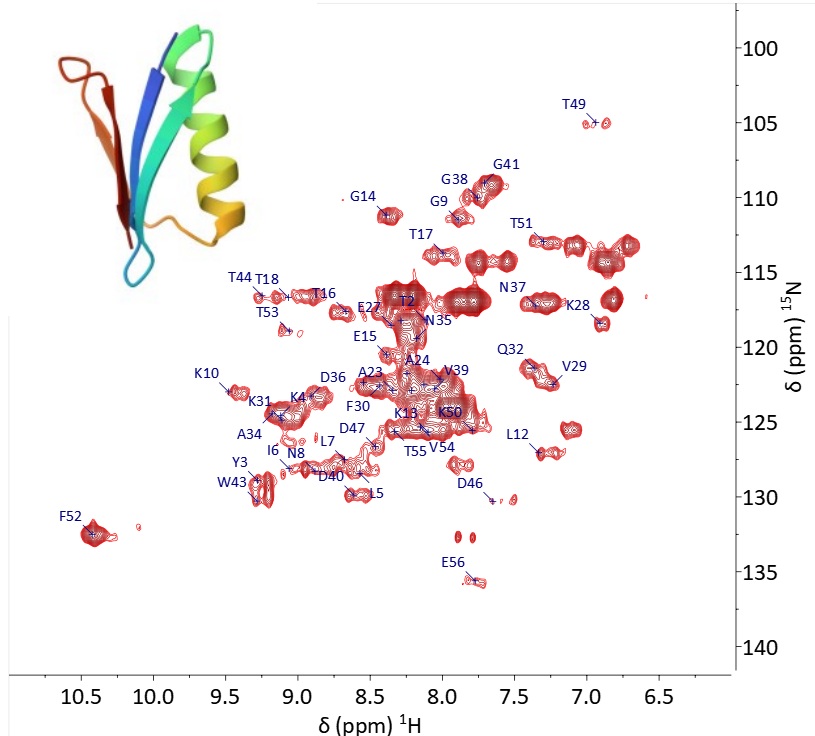
Figure 1. 1H-15N HSQC of 15N-labelled GB1 measured in 9h on a Spinsolve at 80 MHz. The 2D spectrum shows the assignment of the signals for the different amino acids. The structure of the GB1 protein is shown on the top left. Protein structures and their interactions with other proteins or small molecules such as …
App Note – 1H-15N HSQC of GB1 protein measured at 80 MHz Read More »